
Antioxidant BHT 264
CAS:128-37-0
Purity:99%
Contact Now
We will contact you as soon as possible
Your Location:Home >Products >Industrial Chemicals >7078-98-0

Product Details
|
Flammability and Explosibility |
Nonflammable |
InChI:InChI=1/C21H26O/c1-20(2,3)17-13-16(12-15-10-8-7-9-11-15)14-18(19(17)22)21(4,5)6/h7-14H,1-6H3
The isothiourea-catalyzed enantioselecti...
The invention provides a preparation met...
Herein, we report an efficient protocol ...
The first highly enantioselective interm...
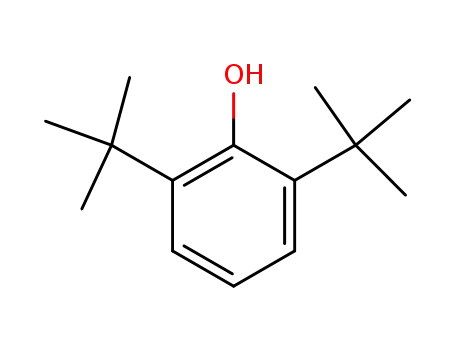
2,6-di-tert-butylphenol

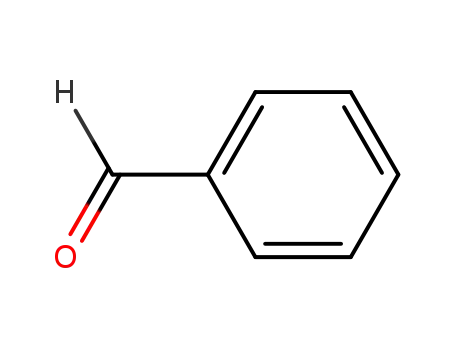
benzaldehyde

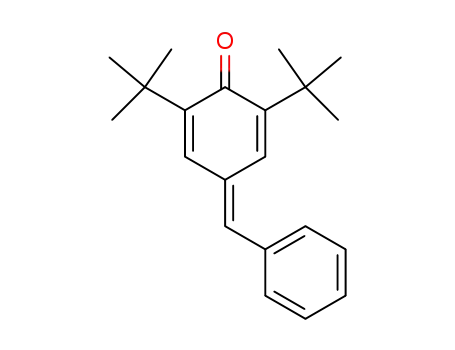
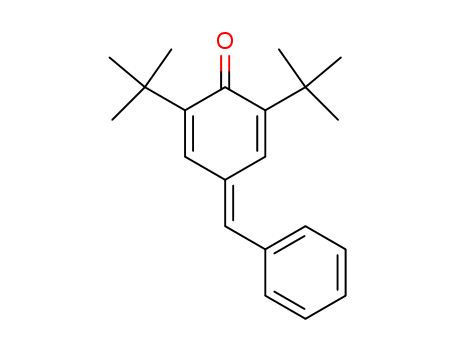
2,6-di-tert-butyl-4-benzylidene-cyclohexa-2,5-dienone
| Conditions | Yield |
|---|---|
|
2,6-di-tert-butylphenol; benzaldehyde;
With
piperidine;
In
toluene;
for 3h;
Reflux;
With
acetic anhydride;
In
toluene;
at 110 - 125 ℃;
for 0.5h;
|
79.1% |
|
2,6-di-tert-butylphenol; benzaldehyde;
With
piperidine;
In
toluene;
Reflux;
With
acetic anhydride;
In
toluene;
for 0.25h;
|
78% |
|
2,6-di-tert-butylphenol; benzaldehyde;
With
piperidine;
In
toluene;
for 19h;
Reflux;
Dean-Stark;
With
acetic anhydride;
In
toluene;
for 1h;
Reflux;
Dean-Stark;
|
78% |
|
With
piperidine; zinc(II) chloride;
In
toluene;
Reagent/catalyst;
Reflux;
Dean-Stark;
|
77.39% |
|
2,6-di-tert-butylphenol; benzaldehyde;
With
piperidine;
In
toluene;
for 7h;
Dean-Stark;
Reflux;
Inert atmosphere;
With
acetic anhydride;
In
toluene;
Dean-Stark;
Heating;
Inert atmosphere;
|
76% |
|
2,6-di-tert-butylphenol; benzaldehyde;
With
piperidine;
In
toluene;
for 13h;
Dean-Stark;
Reflux;
With
acetic anhydride;
In
toluene;
for 0.25h;
Dean-Stark;
|
74% |
|
2,6-di-tert-butylphenol; benzaldehyde;
With
piperidine;
In
toluene;
for 13h;
Reflux;
Dean-Stark;
With
acetic anhydride;
In
toluene;
at 100 ℃;
for 1h;
|
70% |
|
2,6-di-tert-butylphenol; benzaldehyde;
With
piperidine;
In
toluene;
for 3h;
Dean-Stark;
Reflux;
Inert atmosphere;
With
acetic anhydride;
In
toluene;
for 0.25h;
Heating;
Inert atmosphere;
|
69% |
|
2,6-di-tert-butylphenol; benzaldehyde;
With
piperidine;
In
toluene;
at 110 ℃;
for 12h;
Dean-Stark;
With
acetic anhydride;
In
toluene;
at 100 ℃;
for 0.5h;
|
52% |
|
With
piperidine;
In
toluene;
for 13h;
Inert atmosphere;
Reflux;
Dean-Stark;
|
32% |
|
With
piperidine;
In
butan-1-ol;
for 24h;
Reflux;
|
|
|
2,6-di-tert-butylphenol; benzaldehyde;
In
toluene;
for 4h;
Dean-Stark;
Reflux;
Alkaline conditions;
With
acetic anhydride;
In
toluene;
for 0.25h;
Dean-Stark;
|
|
|
With
piperidine;
In
toluene;
for 4h;
Dean-Stark;
Reflux;
|
|
|
2,6-di-tert-butylphenol; benzaldehyde;
With
piperidine;
In
toluene;
at 140 ℃;
for 13h;
Dean-Stark;
Inert atmosphere;
Heating;
With
acetic anhydride;
In
toluene;
for 0.25h;
Dean-Stark;
Inert atmosphere;
Heating;
|
|
|
With
pyridine; acetic anhydride;
In
toluene;
for 4h;
Reflux;
Dean-Stark;
|
|
|
2,6-di-tert-butylphenol; benzaldehyde;
In
toluene;
for 1h;
Reflux;
Inert atmosphere;
Dean-Stark;
With
piperidine;
In
toluene;
for 5h;
Reflux;
Inert atmosphere;
Dean-Stark;
|
|
|
With
piperidine;
In
toluene;
for 4h;
Dean-Stark;
Reflux;
|
|
|
2,6-di-tert-butylphenol; benzaldehyde;
With
piperidine;
In
toluene;
for 4h;
Dean-Stark;
Reflux;
With
acetic anhydride;
In
toluene;
for 0.25h;
|
|
|
2,6-di-tert-butylphenol; benzaldehyde;
With
piperidine;
In
toluene;
for 4h;
Reflux;
With
acetic anhydride;
In
toluene;
at 20 ℃;
for 0.25h;
|
|
|
2,6-di-tert-butylphenol; benzaldehyde;
With
piperidine;
In
toluene;
for 3h;
Reflux;
Dean-Stark;
With
acetic anhydride;
In
toluene;
for 0.25h;
Heating;
|
|
|
2,6-di-tert-butylphenol; benzaldehyde;
In
toluene;
for 1h;
Reflux;
Dean-Stark;
With
piperidine;
In
toluene;
for 3h;
Reflux;
Dean-Stark;
With
acetic anhydride;
In
toluene;
for 0.25h;
|
|
|
2,6-di-tert-butylphenol; benzaldehyde;
With
piperidine;
In
toluene;
for 24h;
Reflux;
With
acetic anhydride;
In
toluene;
for 0.25h;
|
|
|
Multi-step reaction with 2 steps
1: 0.5 h / 120 °C / Microwave irradiation
2: acetic anhydride / 0.05 h / 120 °C / Microwave irradiation; Green chemistry
With
acetic anhydride;
1: |Mannich Aminomethylation;
|
|
|
2,6-di-tert-butylphenol; benzaldehyde;
With
di-n-propylamine;
In
toluene;
Dean-Stark;
Reflux;
With
acetic anhydride;
In
toluene;
for 0.25h;
|
|
|
2,6-di-tert-butylphenol; benzaldehyde;
With
piperidine;
In
toluene;
Dean-Stark;
Reflux;
With
acetic anhydride;
In
toluene;
for 0.5h;
Dean-Stark;
Reflux;
|
|
|
2,6-di-tert-butylphenol; benzaldehyde;
With
piperidine;
In
toluene;
for 12h;
Reflux;
Inert atmosphere;
With
acetic anhydride;
In
toluene;
for 0.25h;
Inert atmosphere;
|
|
|
2,6-di-tert-butylphenol; benzaldehyde;
In
toluene;
Reflux;
With
piperidine;
In
toluene;
Reflux;
With
acetic anhydride;
In
toluene;
for 0.25h;
|
|
|
With
piperidine;
In
toluene;
at 150 ℃;
Dean-Stark;
|
|
|
2,6-di-tert-butylphenol; benzaldehyde;
With
piperidine;
In
toluene;
at 110 ℃;
With
acetic anhydride;
In
toluene;
for 0.25h;
Reflux;
|
|
|
2,6-di-tert-butylphenol; benzaldehyde;
In
toluene;
for 4h;
Dean-Stark;
Reflux;
With
acetic anhydride;
In
toluene;
for 0.25h;
Dean-Stark;
|
|
|
With
piperidine;
In
toluene;
for 12h;
Dean-Stark;
Reflux;
|
|
|
2,6-di-tert-butylphenol; benzaldehyde;
With
piperidine;
In
toluene;
for 4h;
Dean-Stark;
Reflux;
With
acetic anhydride;
In
toluene;
for 0.25h;
Dean-Stark;
|
|
|
2,6-di-tert-butylphenol; benzaldehyde;
With
piperidine;
In
toluene;
Dean-Stark;
Reflux;
With
acetic anhydride;
In
toluene;
for 0.5h;
Reflux;
Dean-Stark;
|
|
|
2,6-di-tert-butylphenol; benzaldehyde;
With
piperidine;
In
toluene;
Dean-Stark;
Reflux;
With
acetic anhydride;
In
toluene;
for 0.5h;
Dean-Stark;
|
|
|
2,6-di-tert-butylphenol; benzaldehyde;
With
piperidine;
In
toluene;
at 140 ℃;
Dean-Stark;
With
acetic anhydride;
In
toluene;
for 0.25h;
Dean-Stark;
Heating;
|
|
|
2,6-di-tert-butylphenol; benzaldehyde;
With
pyrrolidine;
In
toluene;
at 110 ℃;
for 3h;
With
butanoic acid anhydride;
In
toluene;
for 1h;
|
|
|
With
piperidine;
In
toluene;
at 120 ℃;
for 12h;
|
|
|
2,6-di-tert-butylphenol; benzaldehyde;
With
piperidine;
In
toluene;
for 13h;
Reflux;
With
acetic anhydride;
In
toluene;
for 0.25h;
|
|
|
2,6-di-tert-butylphenol; benzaldehyde;
With
piperidine;
In
toluene;
for 6h;
Heating;
Reflux;
With
acetic anhydride;
for 0.5h;
|
|
|
2,6-di-tert-butylphenol; benzaldehyde;
With
piperidine;
In
toluene;
at 140 ℃;
for 1h;
Dean-Stark;
With
acetic anhydride;
In
toluene;
for 0.25h;
Dean-Stark;
|
2,6-di-tert-butyl-4-(phenyl-piperidin-1-yl-methyl)-phenol


2,6-di-tert-butyl-4-benzylidene-cyclohexa-2,5-dienone
| Conditions | Yield |
|---|---|
|
With
acetic anhydride;
at 120 ℃;
for 0.05h;
Microwave irradiation;
Green chemistry;
|
70.18% |
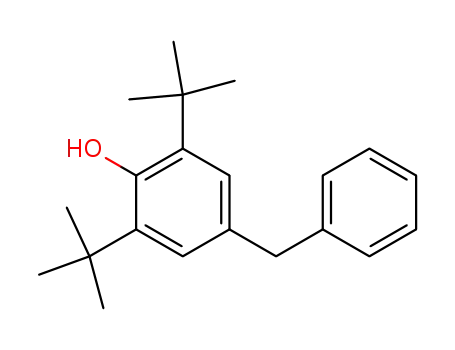
4-benzyl-2,6-di-tert-butylphenol
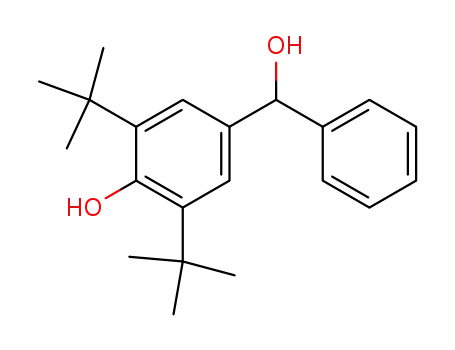
(3,5-di-tert-butyl-4-hydroxyphenyl)phenylcarbinol
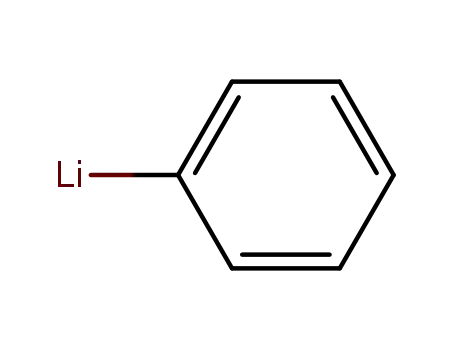
phenyllithium
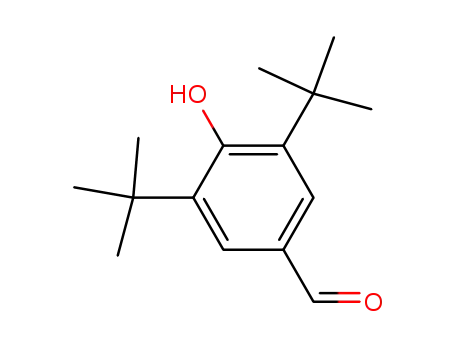
3,5-di-t-butyl-4-hydroxybenzaldehyde
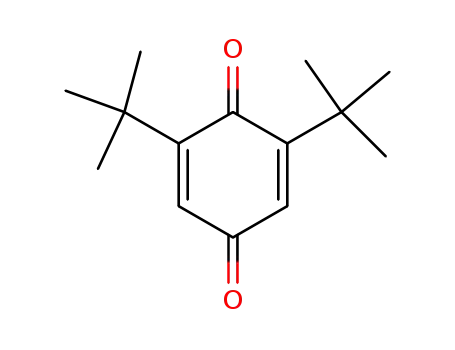
2,6-Di-tert-butyl-1,4-benzoquinone
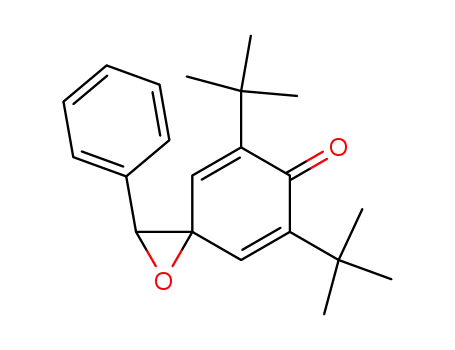
5,7-Di-tert-butyl-2-phenyl-1-oxa-spiro[2.5]octa-4,7-dien-6-one

benzaldehyde
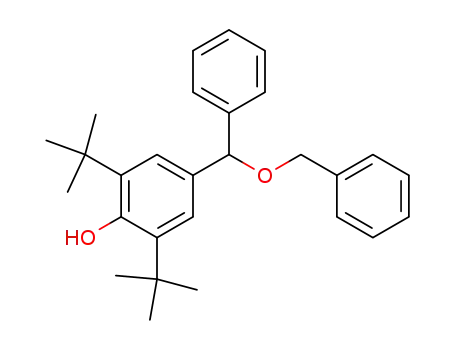
4-((benzyloxy)(phenyl)methyl)-2,6-di-tert-butylphenol