
Antioxidant BHT 264
CAS:128-37-0
Purity:99%
Contact Now
We will contact you as soon as possible
Your Location:Home >Products >High-Tech New Material >5593-70-4
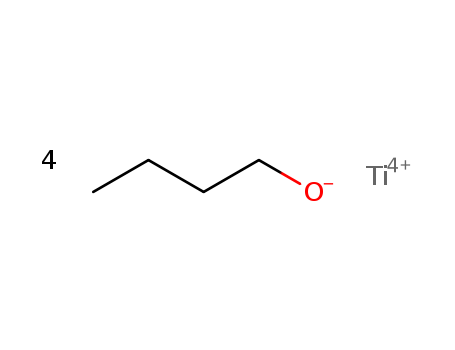

Product Details
|
Air & Water Reactions |
Soluble in water. Reacts with water to form butanol and titanium dioxide, the reaction is not generally thought to be hazardous. |
|
Health Hazard |
LIQUID: Irritating to skin and eyes. If swallowed will cause nausea and vomiting. |
|
Fire Hazard |
Combustible. Containers may explode in fire. May give off dense white smoke. Containers may explode. |
|
Flammability and Explosibility |
Flammable |
|
Safety Profile |
Suspected carcinogen. A poison by intravenous route. Moderately toxic by ingestion. See n-BUTYL ALCOHOL and TITANIUM COMPOUNDS. Flammable when exposed to heat or flame. To fight fire, use water, spray, foam, dry chemical. Incompatible with oxidizing materials. When heated to decomposition it emits acrid and irritating fumes. |
|
Purification Methods |
Dissolve it in *C6H6, filter if solid is present, evaporate and vacuum fractionate through a Widmer 24inch column (p 11). The ester hydrolyses when exposed to air to give hydrated ortho-titanic acid. The titanium content can be determined thus: weigh a sample (ca 0.25g) into a weighed crucible and cover it with 10mL of H2O and a few drops of conc HNO3. Heat (hot plate) carefully till most of the H2O has evaporated. Cool and add more H2O (10mL) and conc HNO3 (2mL), and evaporate carefully (no spillage) to dryness and ignite the residue at 600-650o/1hour. Weigh the residual TiO2. [Bradley et al. J Chem Soc 2773 1952, Speer J Org Chem 14 655 1949, Beilstein 1 II 398, 1 III 1515, 1 IV 1415.] |
|
General Description |
A water-white to pale-yellow liquid with an alcohol-like odor. About the same density as water. Flash point 170°F. Contact may irritate or cause burns. |
InChI:InChI=1/4C4H9O.Ti/c4*1-2-3-4-5;/h4*2-4H2,1H3;/q4*-1;+4/rC16H36O4Ti/c1-5-9-13-17-21(18-14-10-6-2,19-15-11-7-3)20-16-12-8-4/h5-16H2,1-4H3
Interactions in the Mg(OR)2-Ti(OR)4-ROH ...
The authors have carried out an investig...
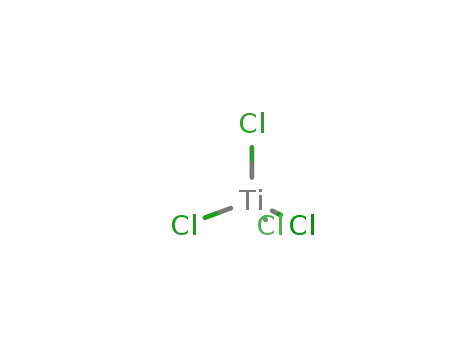
titanium tetrachloride

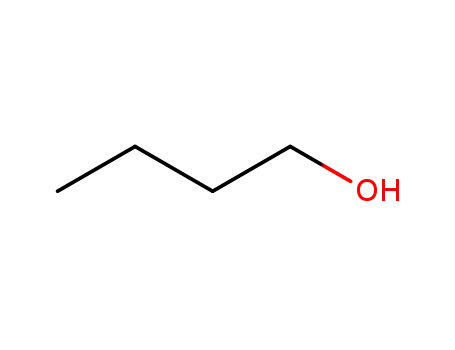
butan-1-ol

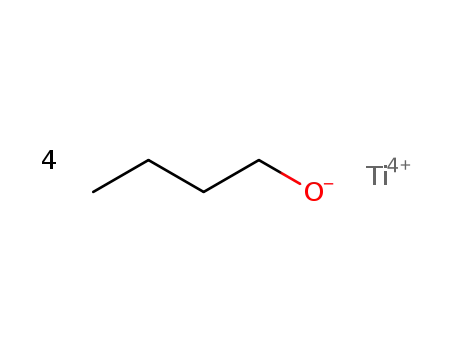
titanium (IV) butoxide
| Conditions | Yield |
|---|---|
|
With
NH4NO3-NH3;
In
toluene;
soln. of TiCl4 added to alcohol with cooling and stirring, soln. added to NH4NO3-NH3 with stirring and cooling at 0-15°C; organic layer separated, solvent removed, product distilled under vacuum;
|
86% |
|
With
formamide;
In
butan-1-ol;
byproducts: NH4Cl; soln. of TiCl4 added to alcohol with cooling and stirring, HCONH2 added, mixture boiled with stirring for 5-6 h, cooled; NH4Cl separated by filtration or HCONH2 added with stirring, product distilled under vacuum;
|
66% |
|
With
formamide;
In
n-heptane;
byproducts: NH4Cl; soln. of TiCl4 added to alcohol with cooling and stirring, HCONH2 added, mixture boiled with stirring for 5-6 h, cooled; NH4Cl separated by filtration or HCONH2 added with stirring, product distilled under vacuum;
|
60% |

butan-1-ol


titanium (IV) butoxide
| Conditions | Yield |
|---|---|
|
With
ammonia;
|
|
|
With
sodium; benzene;
|
|
|
With
ammonia;
|

butan-1-ol
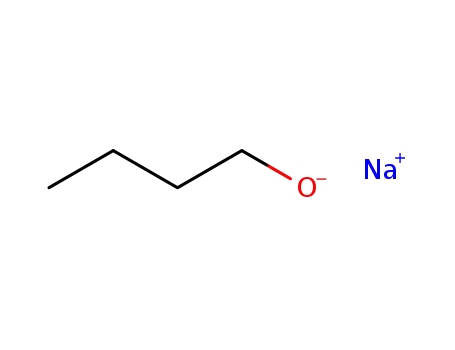
sodium butanolate
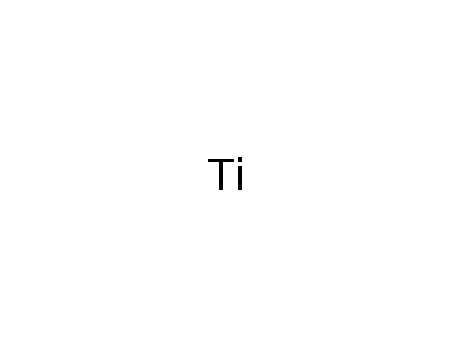
titanium

titanium tetrachloride
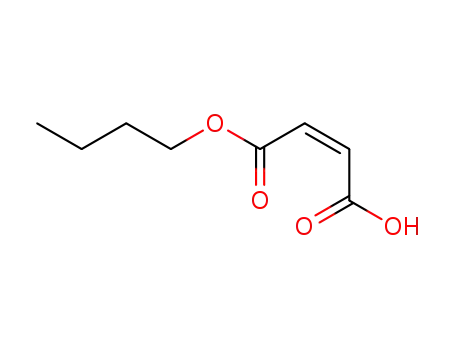
mon-n-butyl maleate
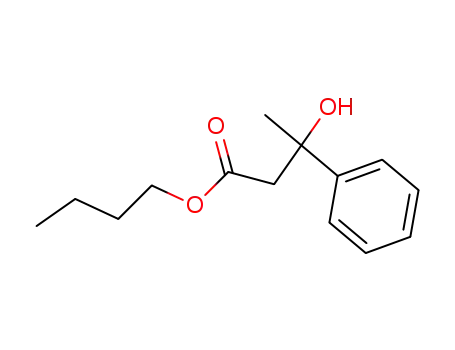
3-Hydroxy-3-phenyl-butyric acid butyl ester
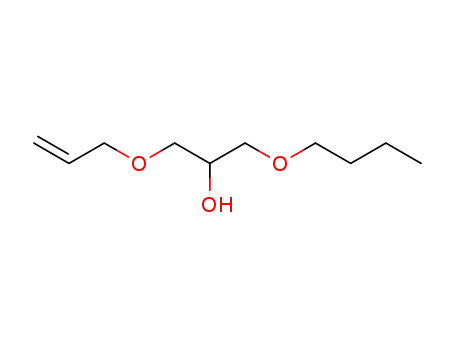
1-allyloxy-3-butoxypropan-2-ol
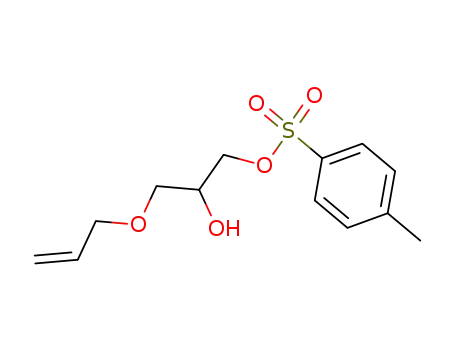
2-hydroxy-3-allyloxypropyl 4-toluenesulfonate