
Antioxidant BHT 264
CAS:128-37-0
Purity:99%
Contact Now
We will contact you as soon as possible
Your Location:Home >Products >Pharmaceutical API >584-85-0
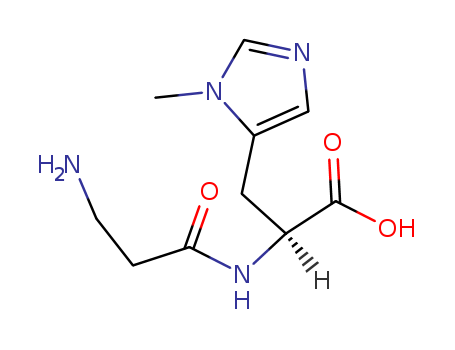

Product Details
|
Purification Methods |
Crystallise anserine from aqueous EtOH. It is hygroscopic and is best stored as the nitrate salt (see below). Purify it by shaking the nitrate salt with Dowex 3 (x4 free base) and washing with H2O, evaporating the filtrate and removing H2O by 3 distillations with 10mL of propan-2-ol. Dissolve the crystals in MeOH and add H2O dropwise until one phase is obtained and cool. Dry the crystals at 60o over P2O5 in a vacuum. The picrate has m 145o (from H2O). [Rinderknecht et al. J Org Chem 29 1968 1964, Beilstein 25 II 408, 25 IV 4383.] |
InChI:InChI=1/C10H16N4O3/c1-14-6-12-5-7(14)4-8(10(16)17)13-9(15)2-3-11/h5-6,8H,2-4,11H2,1H3,(H,13,15)(H,16,17)/t8-/m0/s1
To date, the synthesis of L-anserine has...
The invention provides an anserine inter...
The invention provides preparation metho...
The invention provides two preparation m...
A concise synthesis of anserine and rela...
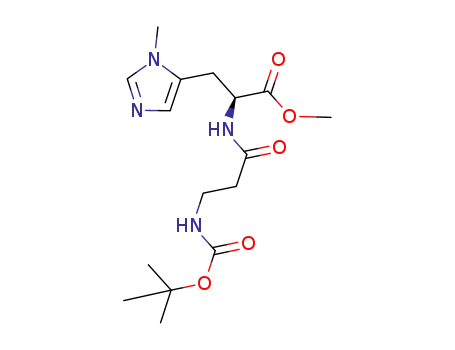
N(α)-(3-((tert-butoxycarbonyl)amino)propylcarbonyl)-N(τ)-methyl-L-histidine methyl ester

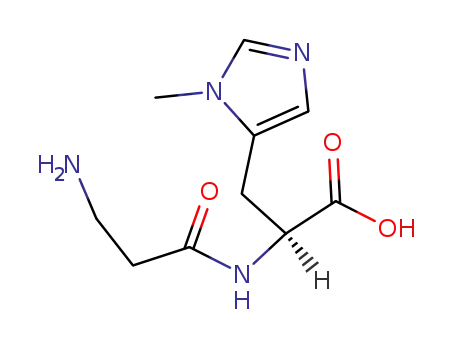
L-anserine
| Conditions | Yield |
|---|---|
|
N(α)-(3-((tert-butoxycarbonyl)amino)propylcarbonyl)-N(τ)-methyl-L-histidine methyl ester;
With
sodium hydroxide;
In
methanol; water;
at 20 ℃;
for 16h;
pH=> 11;
With
hydrogenchloride;
In
methanol; water;
for 15h;
pH=< 3;
|
67% |
|
With
sodium hydroxide;
at 20 ℃;
|
|
|
Multi-step reaction with 2 steps
1: sodium hydroxide; water / methanol / 6 h / 20 - 30 °C
2: hydrogenchloride / water / 4 h / 20 - 50 °C
With
hydrogenchloride; water; sodium hydroxide;
In
methanol; water;
|
|
|
Multi-step reaction with 2 steps
1: hydrogenchloride / methanol; water / 4 h / 20 - 40 °C
2: potassium hydroxide / 8 h / 20 - 30 °C
With
hydrogenchloride; potassium hydroxide;
In
methanol; water;
|
C15H24N4O5


L-anserine
| Conditions | Yield |
|---|---|
|
With
hydrogenchloride;
In
water;
at 20 - 50 ℃;
for 4h;
|
97% |
|
With
hydrogenchloride;
In
water;
at 40 - 50 ℃;
for 8h;
|
5.1 g |
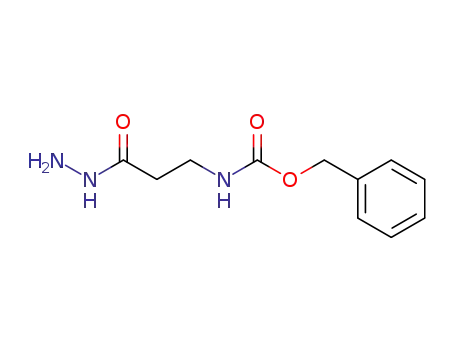
benzyl N-[2-(hydrazinecarbonyl)ethyl]carbamate
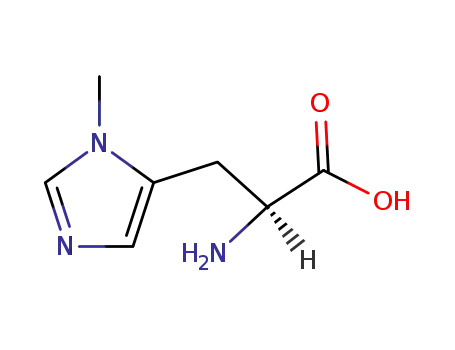
3-methyl-L-histidine
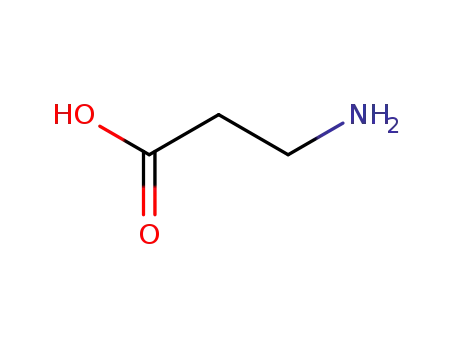
3-amino propanoic acid
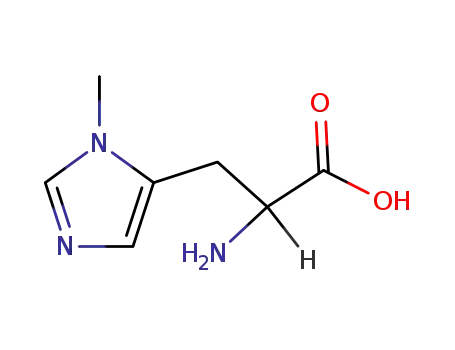
3-methylhistidine

3-methylhistidine

3-amino propanoic acid