
Antioxidant BHT 264
CAS:128-37-0
Purity:99%
Contact Now
We will contact you as soon as possible
Your Location:Home >Products >Intermediates >132-75-2


Product Details
|
Preparation |
Synthesis of 1-naphthylacetonitrile: 695g of 1-chloromethyl naphthalene, 1500ml of methanol and 475ml of water, 350g of sodium cyanide mixture boiling reflux, stirring the reaction for 2h. 1300ml of methanol was evaporated, cooled, and the reactants were washed with water to neutral to obtain 670-680g of 1-naphthylacetonitrile. |
|
Synthesis Reference(s) |
Chemical and Pharmaceutical Bulletin, 39, p. 3030, 1991 DOI: 10.1248/cpb.39.3030 |
|
Application |
1-Naphthylacetonitrile is an organic synthesis intermediate. It is used in the synthesis of 1-naphthylacetate. |
InChI:InChI=1/C12H9N/c13-9-8-11-6-3-5-10-4-1-2-7-12(10)11/h1-7H,8H2
-
Metal-catalyzed silylative dehydration o...
Four new fluorescent dyes derived from t...
The reaction of various alcohols with cy...
(Chemical Equation Presented) A new, mil...
-
-
The synthesis of nitrile under mild cond...
The invention provides a method for dehy...
The invention discloses C labeled α - na...
α-(Hetero)aryl nitriles are important st...
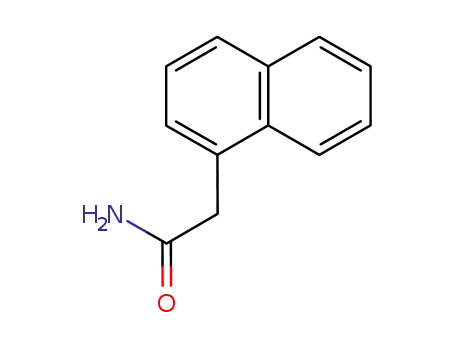
1-Naphthylacetamide


naphthalen-1-ylacetonitrile
| Conditions | Yield |
|---|---|
|
With
trichloromethyl chloroformate;
In
various solvent(s);
0-5 deg C then heated to 60 deg C, 5 min;
|
96% |
|
With
(dimethoxy)methylsilane; copper diacetate; 1,2-bis-(dicyclohexylphosphino)ethane;
In
tetrahydrofuran;
at 20 ℃;
for 12h;
Sealed tube;
|
96% |
|
With
phosgene;
In
tetrahydrofuran; toluene;
at 25 ℃;
for 4h;
Inert atmosphere;
|
91% |
|
With
lead acetate;
In
dichloromethane;
for 12h;
Reflux;
|
90% |
|
With
1,3,5-trichloro-2,4,6-triazine;
In
N,N-dimethyl-formamide;
for 14h;
Ambient temperature;
|
85% |
|
With
1,3,5-trichloro-2,4,6-triazine;
In
N,N-dimethyl-formamide;
for 14h;
Product distribution;
Ambient temperature;
also in dioxan, other temperature, other time;
|
85% |
|
With
C20H25Cl2CoN3; sodium triethylborohydride;
In
toluene;
at 60 ℃;
for 6h;
Inert atmosphere;
|
85% |
|
With
ammonium sulfamate salt;
|
|
|
palladium dichloride;
In
water; acetonitrile;
at 20 ℃;
for 16h;
|
80 % Chromat. |
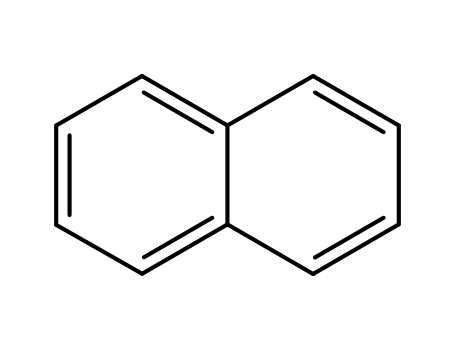
naphthalene

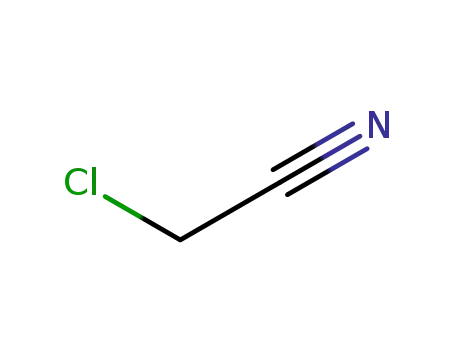
chloroacetonitrile


naphthalen-1-ylacetonitrile
| Conditions | Yield |
|---|---|
|
With
iron(III) chloride;
at 175 ℃;
|
|
|
With
iron(III) oxide; potassium bromide;
|

1-Naphthylacetamide

naphthalene

chloroacetonitrile
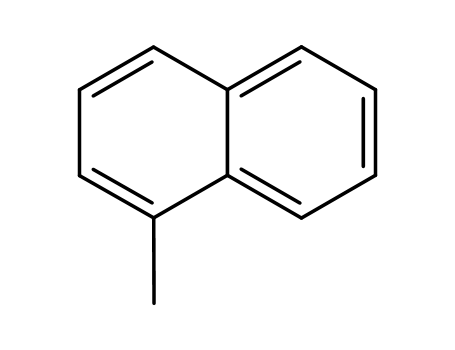
1-Methylnaphthalene
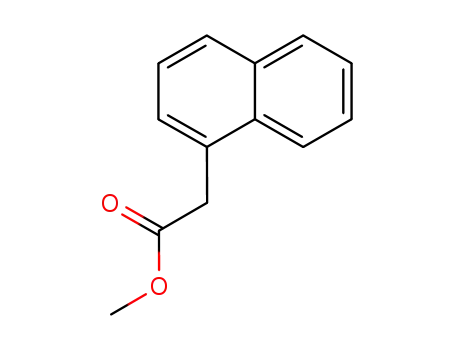
methyl 1-naphthylacetate
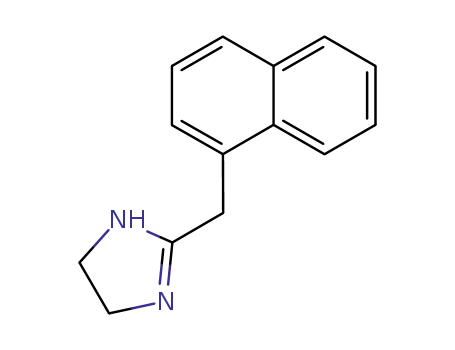
naphazoline
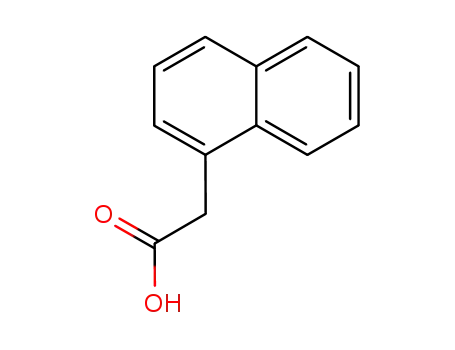
naphth-1-yl acetic acid

1-Naphthylacetamide