
Antioxidant BHT 264
CAS:128-37-0
Purity:99%
Contact Now
We will contact you as soon as possible
Your Location:Home >Products >Pharmaceutical API >74163-84-1

Product Details
|
Pharmacokinetics |
Troparil is a few times more potent than cocaine as a dopamine reuptake inhibitor, but is less potent as a serotonin reuptake inhibitor, and has a duration spanning a few times longer, since the phenyl ring is directly connected to the tropane ring through a non-hydrolyzable carbon-carbon bond. The lack of an ester linkage removes the local anesthetic action from the drug, so troparil is a pure stimulant. This change in activity also makes troparil slightly less cardiotoxic than cocaine. |
InChI:InChI=1/C16H21NO2/c1-17-12-8-9-14(17)15(16(18)19-2)13(10-12)11-6-4-3-5-7-11/h3-7,12-15H,8-10H2,1-2H3/t12-,13+,14+,15-/m0/s1
Introduction Present data indicate that ...
A series of 3β-(p-substituted phenyl)tro...
-
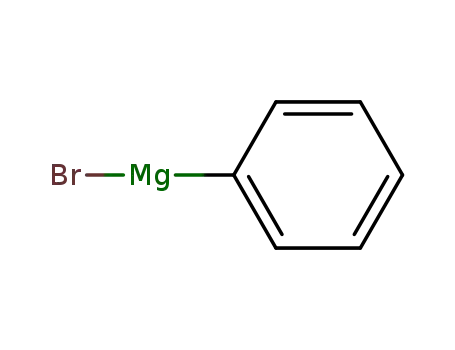
phenylmagnesium bromide

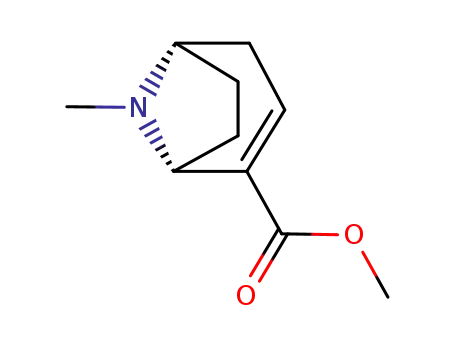
methyl ecgonidine

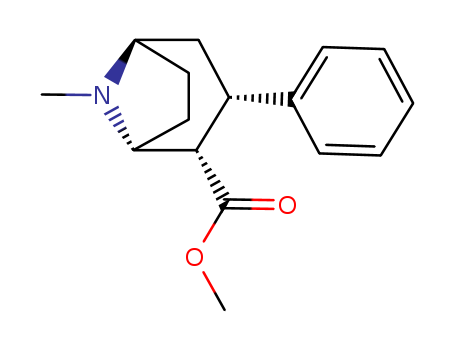
![(1R,3S,5S)-8-Methyl-3-phenyl-8-aza-bicyclo[3.2.1]octane-2-carboxylic acid methyl ester](/upload/2025/1/0563f07d-cd2c-4c73-8763-529fea6a8f8d.png)
(1R,3S,5S)-8-Methyl-3-phenyl-8-aza-bicyclo[3.2.1]octane-2-carboxylic acid methyl ester
| Conditions | Yield |
|---|---|
|
phenylmagnesium bromide; methyl ecgonidine;
In
diethyl ether; dichloromethane;
at -78 - -40 ℃;
for 3.5h;
Inert atmosphere;
With
trifluoroacetic acid;
In
diethyl ether; dichloromethane;
Inert atmosphere;
|
65.28% |
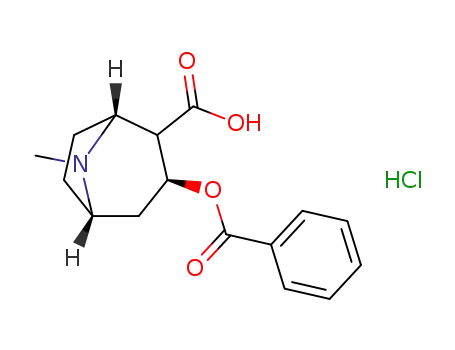
cocaine HCl

![(1R,3S,5S)-8-Methyl-3-phenyl-8-aza-bicyclo[3.2.1]octane-2-carboxylic acid methyl ester](/upload/2025/1/0563f07d-cd2c-4c73-8763-529fea6a8f8d.png)
(1R,3S,5S)-8-Methyl-3-phenyl-8-aza-bicyclo[3.2.1]octane-2-carboxylic acid methyl ester
| Conditions | Yield |
|---|---|
|
Multi-step reaction with 2 steps
1.1: hydrogenchloride / 6 h / Reflux
1.2: 4 h / Reflux; Inert atmosphere
1.3: -40 - 20 °C
2.1: dichloromethane; diethyl ether / 3.5 h / -78 - -40 °C / Inert atmosphere
2.2: Inert atmosphere
With
hydrogenchloride;
In
diethyl ether; dichloromethane;
|
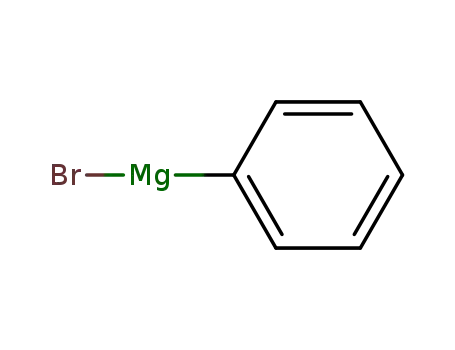
phenylmagnesium bromide

methyl ecgonidine
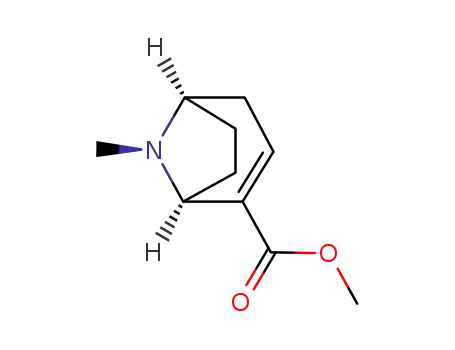
(R)-(-)-anhydroecgonine methyl ester
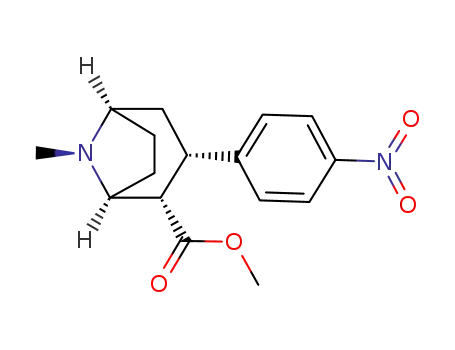
3β-(4-nitrophenyl)tropane-2β-carboxylic acid methyl ester
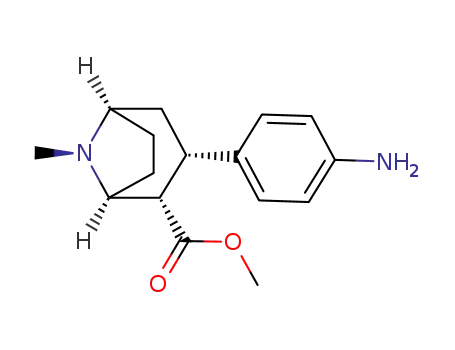
3β-(4-aminophenyl)tropane-2β-carboxylic acid methyl ester
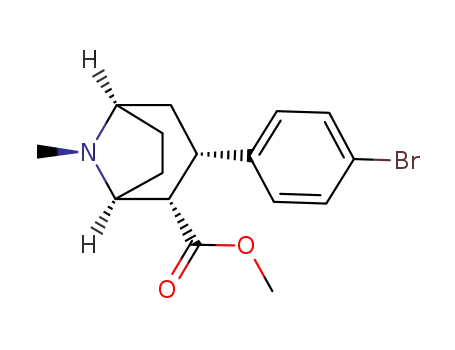
3β-(4-bromophenyl)tropane-2β-carboxylic acid methyl ester
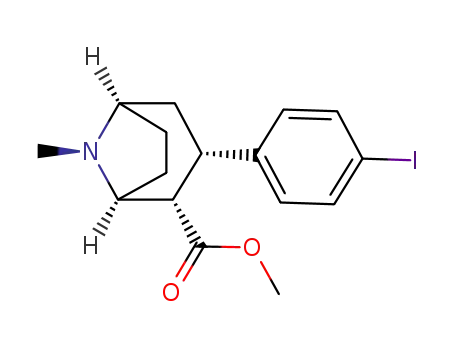
3β-(4-iodophenyl)tropane-2β-carboxylic acid methyl ester