
Antioxidant BHT 264
CAS:128-37-0
Purity:99%
Contact Now
We will contact you as soon as possible
Your Location:Home >Products >Pharmaceutical API >521-18-6

Product Details
|
Manufacturing Process |
A solution of 1.0 g of 3,17-androstandione in 50 ml of methanol and containing 1 g of selenium dioxide, was allowed to remain in an ice-chest overnight. The formed 3,3-dimethoxyandrostan-17-one was not separated. 1 g of solid potassium hydroxide and 2.5 g of sodium borohydride in 2.5 ml of water were added and the mixture allowed to react at room temperature for 24 hours. The solution was then poured into a large excess of water, extracted with methylene chloride, the organic layer dried and evaporated to a residue. The residue was dissolved in ether, and a small amount of selenium removed by filtration. The ether was boiled off and the organic material dissolved in 100 ml of boiling acetone. 25 ml of diluted hydrochloric acid were added, the solution boiled for 5 minutes and then allowed to cool. Upon crystallization, 0.85 g of androstan-17β-ol-3-one was obtained, melting point 175°C to 178°C. |
|
Therapeutic Function |
Androgen |
|
Definition |
ChEBI: Stanolone is a 17beta-hydroxy steroid that is testosterone in which the 4,5 double bond has been reduced to a single bond with alpha-configuration at position 5. It has a role as an androgen, a human metabolite, a Daphnia magna metabolite and a mouse metabolite. It is a 17beta-hydroxy steroid, a 17beta-hydroxyandrostan-3-one and a 3-oxo-5alpha-steroid. It derives from a hydride of a 5alpha-androstane. |
|
General Description |
Dihydrotestosterone (Item No. 15874) is an analytical reference standard categorized as an anabolic androgenic steroid. Anabolic steroids, including dihydrotestosterone, have been used to enhance physical performance in athletes. Dihydrotestosterone is regulated as a Schedule III compound in the United States. This product is intended for research and forensic applications. |
InChI:InChI=1/C19H30O2/c1-18-9-7-13(20)11-12(18)3-4-14-15-5-6-17(21)19(15,2)10-8-16(14)18/h12,14-17,21H,3-11H2,1-2H3/t12?,14-,15-,16-,17-,18-,19-/m0/s1
A kinetic analysis of the 5α-reductases ...
Steroid 5α-reductase (5-AR) catalyses th...
Reduction of the alkenic bond of testost...
Isolated hamster granulosa cells and the...
-
Androgens are essential for male develop...
The invention relates to the field of me...
5α-Dihydrotestosterone (5α-DHT) possesse...
Testosterone and its 5α-reduced form, 5α...
A mild process using a combination of TM...
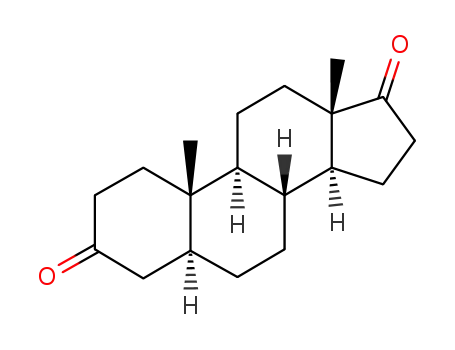
androstanedione

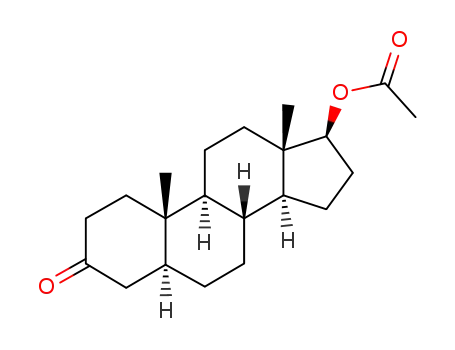
stanolone acetate

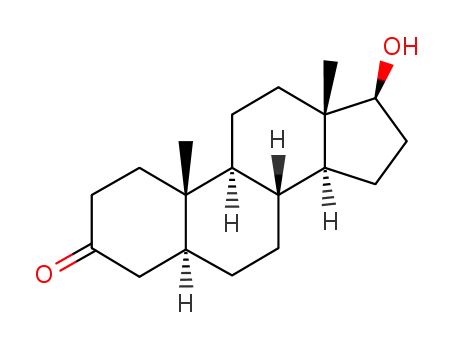
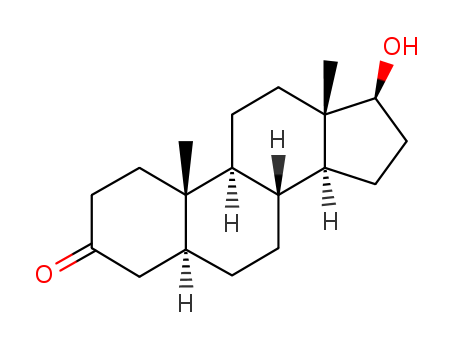
Stanolone
| Conditions | Yield |
|---|---|
|
In
water; dimethyl sulfoxide;
at 25 ℃;
for 240h;
Yield given. Yields of byproduct given;
fermentation with cultures of Cephalosporium aphidicola (IMI 68689);
|
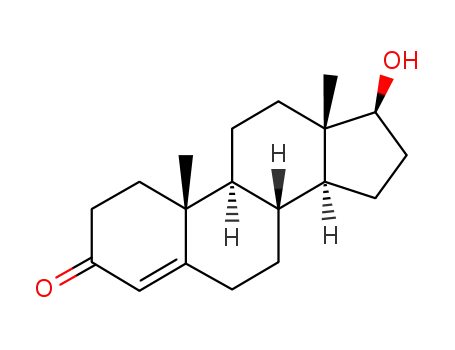
testosterone


Stanolone

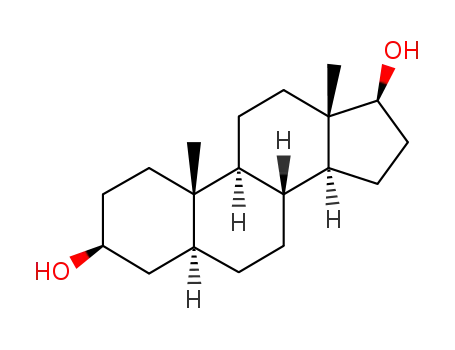
5-androgen-3,17-diol
| Conditions | Yield |
|---|---|
|
With
diethyl ether; palladium;
Hydrogenation;
|

testosterone

androstanedione
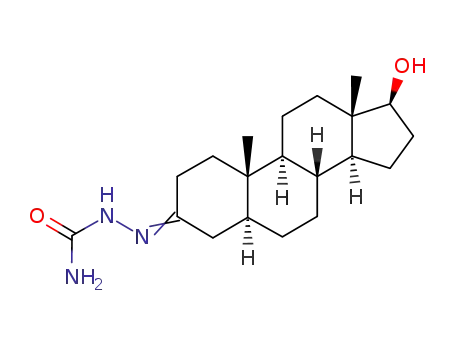
17β-hydroxy-5α-androstan-3-one semicarbazone
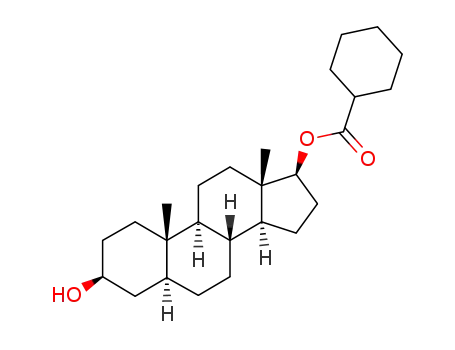
5alpha-Androstane-3beta,17beta-diol-17-hexahydrobenzoate
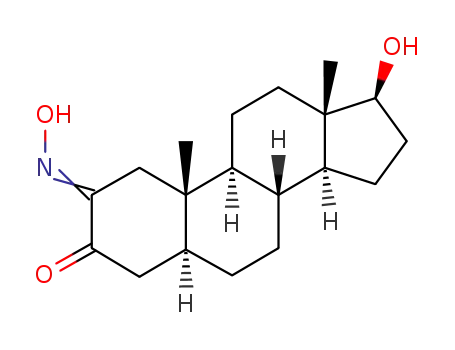
2-Hydroxyimino-17β-hydroxy-androstan-3-on
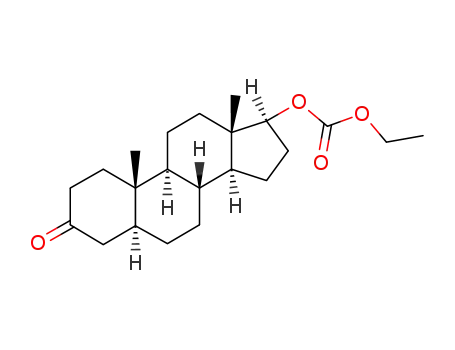
3-Oxo-17β-ethoxycarbonyloxy-5α-androstan
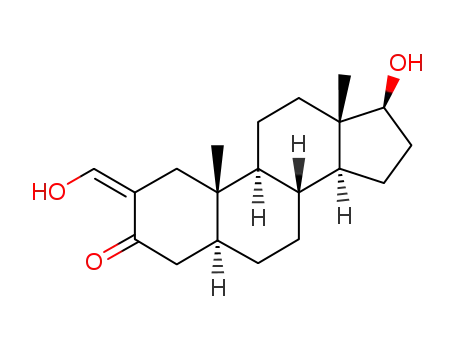
2-hydroxymethylene-17β-hydroxy-5α-androstan-3-one
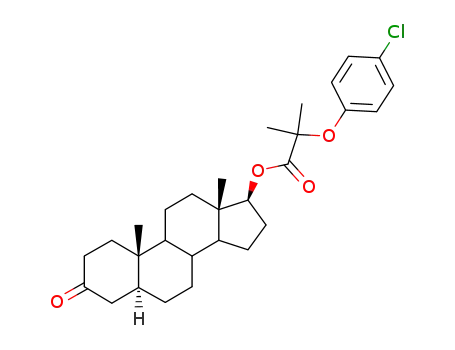
dihydrotestosterone p-chlorophenoxyisobutyrate