
Antioxidant BHT 264
CAS:128-37-0
Purity:99%
Contact Now
We will contact you as soon as possible
Your Location:Home >Products >Pharmaceutical API >56038-13-2


Product Details
|
History |
Sucralose, 1,6-dichloro-1,6-dideoxy-β-D-fructofuranosyl- 4-chloro-4-deoxy-α-D-galactopyranoside, is a trichloro-galactosucrose sweetener developed by the British sugar company Tate & Lyle during the 1970s (U.S. Pat. 4,343,934 (Aug. 10, 1982), M. R. Jenner and D. Waite (to Talres Development), (U.S. Pat. 4,362,869 (Dec. 7, 1982), M. R. Jenner and co-workers (to Talres Development), and (U.S. Pat. 4,435,440 (Mar. 6, 1984), L. Hough, S. P. Phadnis, and R. A. Khan (to Tate & Lyle). It was licensed to McNeil-PPC, Inc., a Johnson & Johnson subsidiary, in the United States until a new agreement took place in February, 2004. McNeil Nutritionals retained ownership of SPLENDA Brand and the right for its worldwide retail and food service business. Tate & Lyle became the sole manufacturer of SPLENDA Brand sucralose and owned the right for its worldwide ingredient sales. |
|
Production Methods |
Sucralose may be prepared by a variety of methods that involve the selective substitution of three sucrose hydroxyl groups by chlorine. Sucralose can also be synthesized by the reaction of sucrose (or an acetate) with thionyl chloride. |
|
Definition |
ChEBI: A disaccharide derivative consisting of 4-chloro-4-deoxy-alpha-D-galactopyranose and 1,6-dichloro-1,6-dideoxy-beta-D-fructofuranose units linked by a glycosidic bond. |
|
General Description |
Certified pharmaceutical secondary standards for application in quality control provide pharma laboratories and manufacturers with a convenient and cost-effective alternative to pharmacopeia primary standards.Sucralose is a polar, chlorinated sugar synthesized from saccharose precursor. It is widely used as a sweetener in a number of food and beverage products. |
|
Pharmaceutical Applications |
Sucralose is used as a sweetening agent in beverages, foods, and pharmaceutical applications. It has a sweetening power approximately 300–1000 times that of sucrose and has no aftertaste. It has no nutritional value, is noncariogenic, does not promote dental caries, and produces no glycemic response. |
|
Biochem/physiol Actions |
A synthetic sweet tastant detectable by humans. Activates T1R2/T1R3 sweet taste receptors on enteroendocrine cells and elicits increased hormonal secretion of glucagon-like peptide-1 and glucose-dependent insulinotrophic peptide. |
|
Safety |
Sucralose is generally regarded as a nontoxic and nonirritant material and is approved, in a number of countries, for use in food products. Following oral consumption, sucralose is mainly unabsorbed and is excreted in the feces. The WHO has set an acceptable daily intake for sucralose of up to 15 mg/kg body-weight. LD50 (mouse, oral): > 16 g/kg LD50 (rat, oral): > 10 g/kg |
|
storage |
Sucralose is a relatively stable material. In aqueous solution, at highly acidic conditions (pH < 3), and at high temperatures (≤35℃), it is hydrolyzed to a limited extent, producing 4-chloro-4- deoxygalactose and 1,6-dichloro-1,6-dideoxyfructose. In food products, sucralose remains stable throughout extended storage periods, even at low pH. However, it is most stable at pH 5–6. Sucralose should be stored in a well-closed container in a cool, dry place, at a temperature not exceeding 21℃. Sucralose, when heated at elevated temperatures, may break down with the release of carbon dioxide, carbon monoxide, and minor amounts of hydrogen chloride. |
|
Regulatory Status |
The FDA, in April 1998, approved sucralose for use as a tabletop sweetener and as an additive in a variety of food products. In the UK, sucralose was fully authorized for use in food products in 2005. It is also accepted for use in many other countries worldwide. Included in the Canadian List of Acceptable Nonmedicinal Ingredients. |
InChI:InChI=1/C12H19Cl3O8/c13-1-4-7(17)10(20)12(3-14,22-4)23-11-9(19)8(18)6(15)5(2-16)21-11/h4-11,16-20H,1-3H2
Bioconversion of sucralose-6-acetate to ...
The invention provides a preparation met...
The invention discloses a recrystallizat...
The invention provides a modified synthe...
The invention discloses a deacylating me...
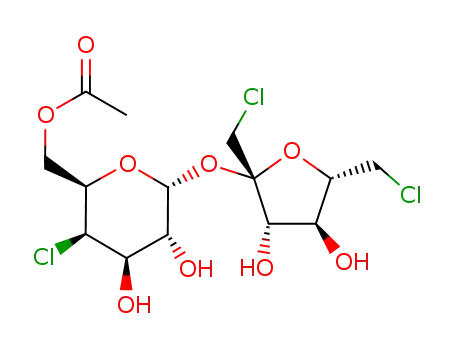
sucralose-6-acetate

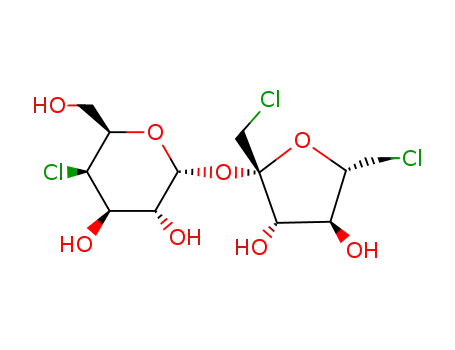
Sucralose
| Conditions | Yield |
|---|---|
|
With
methanol; sodium methylate;
at 40 - 50 ℃;
for 2h;
|
95% |
|
With
methanol; sodium methylate;
at 15 - 20 ℃;
for 5h;
|
86% |
|
4-methyl-morpholine;
In
ethanol;
for 3h;
pH=9.5;
Product distribution / selectivity;
Heating / reflux;
|
72.4% |
|
triethylamine;
In
methanol;
for 7h;
pH=13;
Product distribution / selectivity;
Heating / reflux;
|
70.8% |
|
tert-butylamine;
In
methanol;
at 20 ℃;
for 5h;
pH=12;
Product distribution / selectivity;
|
68% |
|
With
methanol; acetyl chloride;
at 35 - 40 ℃;
for 12 - 16h;
pH=3.5 - 4;
Product distribution / selectivity;
Acetate buffer in methanol;
|
28% |
|
With
sodium hydroxide; water; N,N-dimethylammonium chloride;
pH=~ 10.5;
|
|
|
sucralose-6-acetate;
With
sodium benzoate; sodium hydroxide;
In
water; N,N-dimethyl-formamide;
at 5 - 25 ℃;
pH=11.5 - 13.5;
With
acetic acid;
In
water; N,N-dimethyl-formamide;
at 0 - 5 ℃;
Product distribution / selectivity;
|
|
|
With
calcium hydroxide;
In
water; N,N-dimethyl-formamide;
for 8h;
pH=9.5;
Product distribution / selectivity;
|
|
|
sucralose-6-acetate;
With
methanol; calcium hydroxide; water;
pH=9.5;
With
sulfuric acid; water;
In
methanol;
|
|
|
With
calcium hydroxide; water;
pH=9.0 - 9.5;
Product distribution / selectivity;
|
|
|
|
|
|
sucralose-6-acetate;
With
sodium hydroxide; water;
In
N,N-dimethyl-formamide;
at 33 - 40 ℃;
for 4 - 8h;
pH=10.5 - 11.2;
With
hydrogenchloride; water;
In
N,N-dimethyl-formamide;
for 0.25h;
pH=5.53 - 7;
Product distribution / selectivity;
|
|
|
sucralose-6-acetate;
With
magnesium hydroxide; sodium hydroxide; water;
In
N,N-dimethyl-formamide;
at 40 ℃;
for 3h;
pH=8.95 - 10.5;
With
carbon dioxide; water;
In
N,N-dimethyl-formamide;
for 1.5h;
pH=7.6;
Product distribution / selectivity;
|
|
|
sucralose-6-acetate;
With
sodium hydroxide; water;
In
N,N-dimethyl-formamide;
at 40 ℃;
for 4h;
pH=10.5;
With
carbon dioxide; water;
In
N,N-dimethyl-formamide;
for 0.5h;
pH=7.2 - 7.5;
Product distribution / selectivity;
|
|
|
sucralose-6-acetate;
With
sodium hydroxide; water;
In
N,N-dimethyl-formamide;
at 33.0456 ℃;
for 8h;
pH=11.1;
large scale;
With
hydrogenchloride; water;
In
N,N-dimethyl-formamide;
for 0.25h;
Product distribution / selectivity;
|
|
|
sucralose-6-acetate;
With
calcium hydroxide; water;
In
N,N-dimethyl-formamide;
at 40 ℃;
for 2.5h;
pH=10.2;
With
carbon dioxide; water;
In
N,N-dimethyl-formamide;
pH=5.8 - 6;
Product distribution / selectivity;
|
|
|
In
methanol;
Product distribution / selectivity;
|
|
|
With
sodium methylate;
In
methanol;
at 30 ℃;
for 4h;
|
|
|
With
sodium hydroxide;
In
N,N-dimethyl-formamide;
at 22 ℃;
pH=11.7;
Product distribution / selectivity;
|
|
|
sucralose-6-acetate;
With
water; sodium hydroxide;
In
N,N-dimethyl-formamide;
at 40 ℃;
for 4h;
pH=10.5;
With
hydrogenchloride; water;
In
N,N-dimethyl-formamide;
pH=7;
Product distribution / selectivity;
|
|
|
With
sodium hydroxide;
In
water;
pH=9.0 - 9.5;
|
|
|
With
methanol; ammonia;
Industry scale;
|
|
|
With
sodium hydroxide;
In
water; N,N-dimethyl-formamide;
at 53 ℃;
for 6h;
under 40 Torr;
pH=12.4;
|
|
|
With
methanol; potassium hydroxide;
at 45 ℃;
for 4h;
pH=11;
|
|
|
With
sodium hypochlorite; water;
In
methanol;
at 0 - 5 ℃;
for 4h;
pH=12;
Solvent;
pH-value;
|
48 g |
|
With
Arthrobacter sp. lipase;
In
aq. buffer;
for 96h;
Reagent/catalyst;
Enzymatic reaction;
|
|
|
With
sodium methylate;
In
methanol;
at 20 ℃;
for 4h;
|
7.9 g |
|
With
sodium methylate;
In
methanol;
at 58 - 62 ℃;
for 5h;
Time;
|
141 g |
|
With
sodium hydroxide;
In
methanol;
at 40 ℃;
Temperature;
Flow reactor;
|
|
|
pH=9.0 - 9.5;
Product distribution / selectivity;
|
|
|
With
sodium hydroxide; water;
pH=9.0 - 9.5;
Product distribution / selectivity;
|
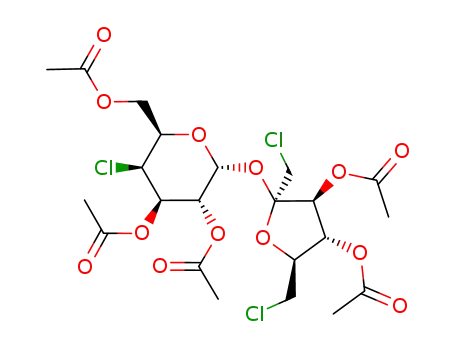
2,3,6-tri-O-acetyl-4-chloro-4-deoxy-α-D-galactopyranosyl 3,4-di-O-acetyl-1,6-dichloro-1,6-dideoxy-β-D-fructofuranoside


Sucralose
| Conditions | Yield |
|---|---|
|
2,3,6-tri-O-acetyl-4-chloro-4-deoxy-α-D-galactopyranosyl 3,4-di-O-acetyl-1,6-dichloro-1,6-dideoxy-β-D-fructofuranoside;
With
methanol; sodium hydroxide; water;
for 0.25h;
In
methanol; water;
Product distribution / selectivity;
|
85% |
|
With
methanol; sodium methylate;
at 15 - 20 ℃;
for 5h;
|
83% |
|
With
methanol; sodium carbonate;
at 20 ℃;
for 2h;
|
70% |
|
With
tetra(n-butyl)ammonium hydroxide;
In
methanol;
at 20 ℃;
pH=9;
|
68.8% |
|
2,3,6-tri-O-acetyl-4-chloro-4-deoxy-α-D-galactopyranosyl 3,4-di-O-acetyl-1,6-dichloro-1,6-dideoxy-β-D-fructofuranoside;
With
methanol; sodium methylate;
at 20 - 45 ℃;
for 1.5h;
With
water; acetic acid;
at 20 ℃;
pH=7.0;
|
|
|
2,3,6-tri-O-acetyl-4-chloro-4-deoxy-α-D-galactopyranosyl 3,4-di-O-acetyl-1,6-dichloro-1,6-dideoxy-β-D-fructofuranoside;
With
methanol; sodium methylate;
pH=10.5;
In
methanol;
|
|
|
2,3,6-tri-O-acetyl-4-chloro-4-deoxy-α-D-galactopyranosyl 3,4-di-O-acetyl-1,6-dichloro-1,6-dideoxy-β-D-fructofuranoside;
With
methanol; sodium methylate;
at 25 - 30 ℃;
Product distribution / selectivity;
|
|
|
2,3,6-tri-O-acetyl-4-chloro-4-deoxy-α-D-galactopyranosyl 3,4-di-O-acetyl-1,6-dichloro-1,6-dideoxy-β-D-fructofuranoside;
With
methanol; sodium methylate;
at 10 - 22 ℃;
for 0.5 - 4h;
pH=8 - 11;
With
acetic acid;
In
methanol;
at 22 ℃;
Product distribution / selectivity;
|
|
|
2,3,6-tri-O-acetyl-4-chloro-4-deoxy-α-D-galactopyranosyl 3,4-di-O-acetyl-1,6-dichloro-1,6-dideoxy-β-D-fructofuranoside;
With
methanol; sodium hydroxide; water;
at 22 ℃;
for 0.583333 - 0.833333h;
pH=8 - 9;
With
acetic acid;
In
methanol; water;
at 22 ℃;
pH=6.5 - 7;
Product distribution / selectivity;
|

sucralose-6-acetate
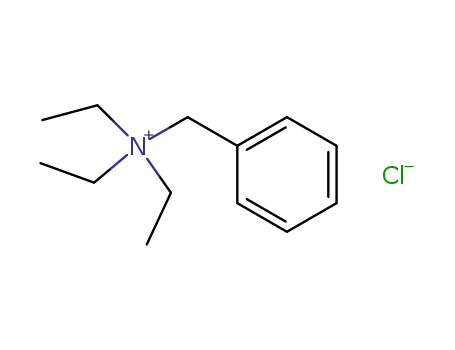
N-benzyl-N,N,N-triethylammonium chloride
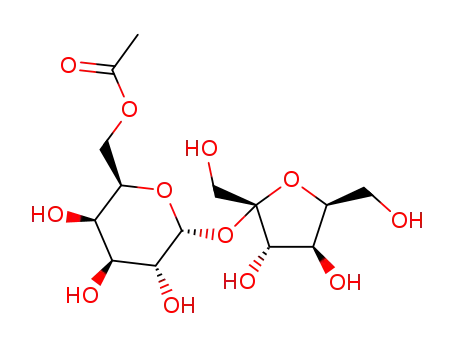
sucrose-6-acetate
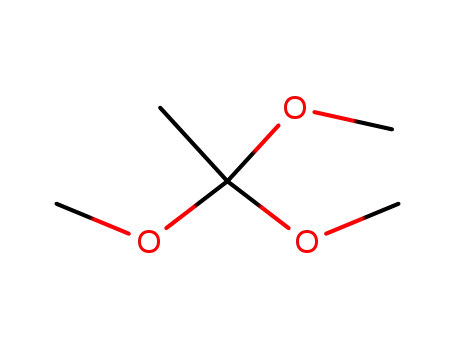
Trimethyl orthoacetate
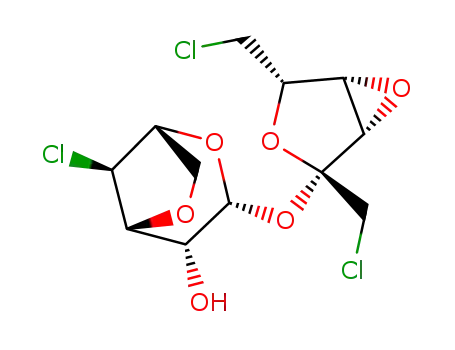
3,6-anhydro-4-chloro-4-deoxy-α-D-galactopyranosyl 3,4-anhydro-1,6-dichloro-1,6-dideoxy-β-D-lyxo-hexulofuranoside
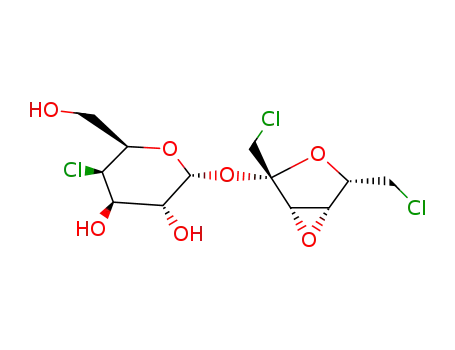
4-chloro-4-deoxy-α-D-galactopyranosyl 3,4-anhydro-1,6-dichloro-1,6-dideoxy-β-D-lyxo-hexulofuranoside
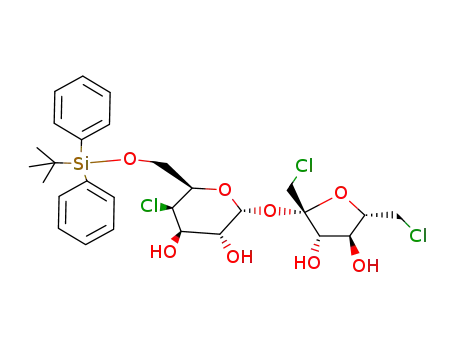
6-O-tert-butyldiphenylsilyl-4-chloro-4-deoxy-α-D-galactopyranosyl 1,6-dichloro-1,6-dideoxy-β-D-fructofuranoside
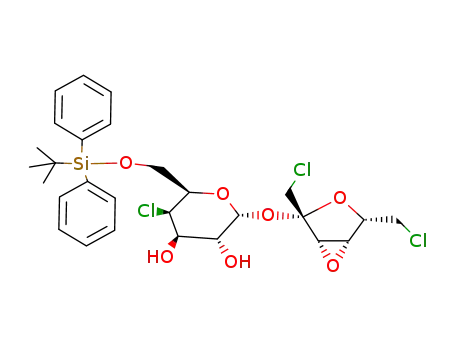
6-O-tert-butyldiphenylsilyl-4-chloro-4-deoxy-α-D-galactopyranosyl 3,4-anhydro-1,6-dichloro-1,6-dideoxy-β-D-lyxo-hexulofuranoside