
Antioxidant BHT 264
CAS:128-37-0
Purity:99%
Contact Now
We will contact you as soon as possible
Your Location:Home >Products >Intermediates >100-61-8
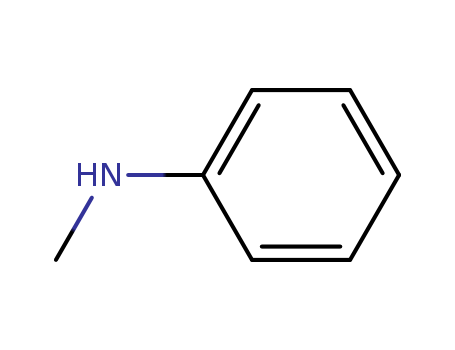
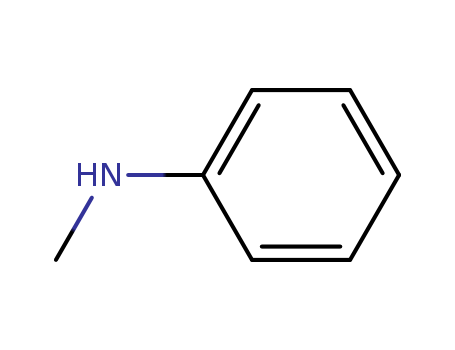
Product Details
|
Physical properties |
Colorless to yellow to pale brown liquid with a faint, ammonia-like odor. Odor threshold concentration is 1.7 ppm (quoted, Amoore and Hautala, 1983). |
|
Definition |
ChEBI: N-methylaniline is a methylaniline that is aniline carrying a methyl substituent at the nitrogen atom. It is a phenylalkylamine, a secondary amine and a methylaniline. It derives from an aniline. |
|
Preparation |
N-methylaniline was synthesized by the reaction of aniline with dimethyl sulfate. Dimethyl sulfate was added dropwise to the mixed solution of aniline and water below 10°C, stirred for 1 h, and then added dropwise with 30% sodium hydroxide solution. The upper layer is the organic phase, and the lower layer is extracted with benzene. After the benzene is recovered from the extract, the obtained oil lookchem-like substance is combined with the organic phase to obtain a mixture of aniline, N-methylaniline and N,N-dimethylaniline. The mixture was treated with sulfuric acid, and the aniline formed sulfate crystals which were filtered off. N,N-dimethylaniline can be converted to N-methylaniline by the following reaction. |
|
Application |
N-Methylaniline (NMA) was used in the preparation of self-assembled poly(N-methylaniline)-lignosulfonate (PNMA-LS) composite spheres with reactive silver-ion adsorbability. NMA was also used in electrodeposition of poly(N-methylaniline) (PNMA) coatings on a steel disc electrode using potentiodynamic, potentiostatic and galvanostatic techniques. |
|
Synthesis Reference(s) |
Chemical and Pharmaceutical Bulletin, 14, p. 1007, 1966 DOI: 10.1248/cpb.14.1007Journal of the American Chemical Society, 107, p. 493, 1985 DOI: 10.1021/ja00288a037Synthetic Communications, 13, p. 601, 1983 DOI: 10.1080/00397918308059535 |
|
General Description |
Chemical oxidation of N-methylaniline with dichromate (oxidant) has been studied by Raman spectroscopy. |
|
Hazard |
Toxic by ingestion, inhalation, and skin absorption. Methemoglobinemia and central nervous system impairment. |
|
Health Hazard |
Recommended Personal Protective Equipment: Approved respirator; rubber gloves; splash proof goggles; Symptoms Following Exposure: Inhalation causes dizziness and headache. Ingestion causes bluish discoloration (cyanosis) of lips, ear lobes, and fingernail beds. Liquid irritates eyes. Absorption through skin produces same symptoms as for ingestion; General Treatment for Exposure: INHALATION: remove victim to fresh air and call a physician at once; administer oxygen until physician arrives. INGESTION: give large amount of water; get medical attention at once. EYES or SKIN: flush with plenty of water for at least 15 min.; if cyanosis is present, shower with soap and warm water, with special attention to scalp and finger nails; remove any contaminated clothing; Toxicity by Inhalation (Threshold Limit Value): Data not available; Short-Term Inhalation Limits: Data not available; Toxicity by Ingestion: Data not available; Late Toxicity: Data not available; Vapor (Gas) Irritant Characteristics: Data not available; Liquid or Solid Irritant Characteristics: Data not available; Odor Threshold: Data not available. |
|
Chemical Reactivity |
Reactivity with Water No reaction; Reactivity with Common Materials: May attack some forms of plastic; Stability During Transport: Stable; Neutralizing Agents for Acids and Caustics: Not pertinent; Polymerization: Not pertinent; Inhibitor of Polymerization: Not pertinent. |
|
Safety Profile |
Poison by ingestion and intravenous routes. Moderately toxic by subcutaneous route. When heated to decomposition it emits toxic fumes of NOx. |
|
Potential Exposure |
The material is used as an intermediate in organic synthesis, as a solvent and as an acid acceptor |
|
Carcinogenicity |
N-methyl aniline (1.95 g/kg of food) given together with sodium nitrite (1.0 g/l of drinking water) to Swiss mice resulted in a 17% incidence of lung adenomas and a 14% incidence of malignant lymphomas; there were no carcinogenic effects in animals treated with Nmethyl aniline alone, suggesting that in vivo nitrosation is necessary for forming carcinogenic nitrosamines.In bacterial mutagenicity assays N-methyl aniline was negative with or without metabolic activation. |
|
Environmental fate |
Soil. Reacts slowly with humic acids or humates forming quinoidal structures (Parris, 1980). |
|
Shipping |
UN2294 N-Methylaniline, Hazard Class: 6.1; Labels: 6.1-Poisonous materials. |
|
Purification Methods |
Dry it with KOH pellets and fractionally distil it under vacuum. Acetylate, and the acetyl derivative is recrystallised to constant melting point (m 101-102o), then hydrolysed with aqueous HCl and distilled from zinc dust under reduced pressure. [Hammond & Parks J Am Chem Soc 77 340 1955, Beilstein 12 IV 241.] |
|
Waste Disposal |
Controlled incineration whereby oxides of nitrogen are removed from the effluent gas by scrubber, catalytic or thermal device. |
InChI:InChI=1/C7H9N/c1-6-4-2-3-5-7(6)8/h2-5H,8H2,1H3
Flash vacuum pyrolysis (FVP) of 3-oxo-2-...
N,N'-disubstituted ureas have been obtai...
Oxygenation of olefin, N-dealkylation of...
Utilization of dioxygen as the terminal ...
At 200 °C and 5 MPa of initial total pre...
Sulfamic acid has been proved to be the ...
β-Amino-β-lactams and their gem-difuncti...
Electrocatalytic oxidation of N-alkyl-N-...
Inhibition of tubulin polymerization is ...
A series of N-heterocyclic carbene-iridi...
The metal-bound ethoxide species that ar...
The addition of triethylamine to a solut...
In an attempt to assess the synthetic ut...
Seven new chelated cyclometalated Ir com...
The NiCl2·2H2O/Li/DTBB (10 mol%) combina...
Direct monomethylation of primary amines...
Alumina catalysts prepared by different ...
Aniline alkylation with methanol on zeol...
-
Hydrogenations using supported metal cat...
Oxidative dimerization of N,N-dimethylan...
The direct reductive N-arylation of nitr...
-
Oxidative N-dealkylation of NN-dimethyla...
The reaction of aniline with methanol in...
-
Tetrahydroquinoline skeletons can be for...
A simple and practical method for the de...
A mesoionic N-heterocyclic olefin (mNHO)...
We report the photo-catalytic N-alkylati...
An active Pd/ZrCuOx catalyst was prepare...
The TiO2/UV photocatalytic degradation o...
Copper-dioxygen adducts are important bi...
Abstract: An excellent catalyst with a l...
The development of high-yielding, "green...
The deprotection of sulfonamides is achi...
Aminoarenes were readily converted into ...
Zn(OAc)2 was found to give excellent cat...
C-Benzotriazole bonds were selectively t...
The convenience and efficiency of using ...
Photolysis of N-nitrosamines in acidic a...
-
-
A new biogenic potentially tetradentate ...
Methylation of secondary amines was achi...
A base-free catalyst system Co(acac)3/BM...
Multicomponent reactions performed on th...
The hydrogenation of dicarboxylic acids ...
In the presence of a Na-exchanged Y fauj...
The BH4-- promoted electrochemical hydro...
Herein a green and convenient catalytic ...
The catalytic activities of a series of ...
Intramolecular hydroarylation-redox cros...
Here we disclose a general Co(II/III/IV)...
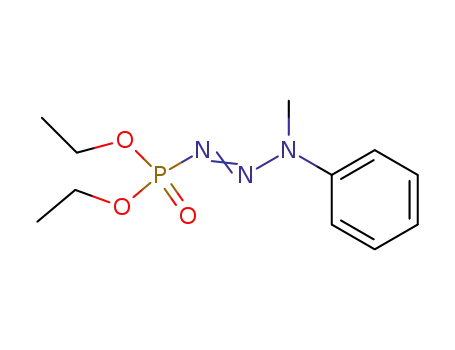
1-Diethoxyphosphonyl-3-methyl-3-phenyltriazen

benzene

ethylbenzene

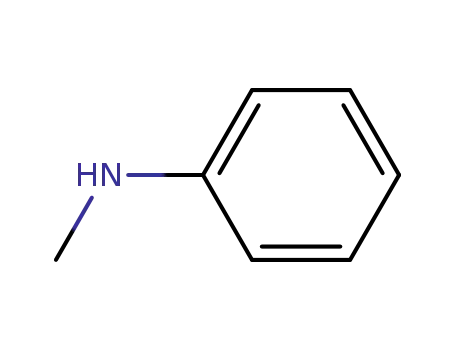
N-methylaniline
| Conditions | Yield |
|---|---|
|
With
aluminium trichloride;
at 20 ℃;
for 16h;
|
1.40 g 1.01 g |
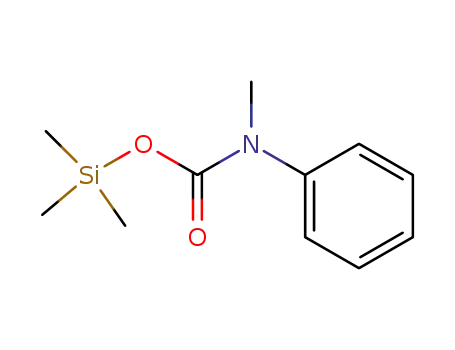
trimethylsilyl N-methyl-N-phenylcarbamate

isopropyl alcohol

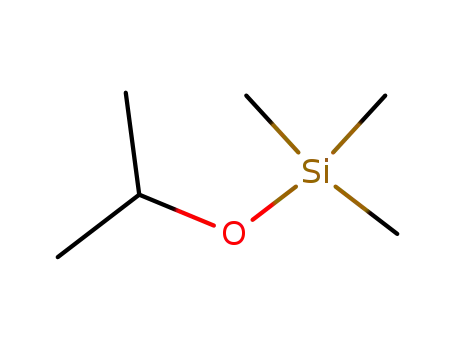
isopropoxytrimethylsilane

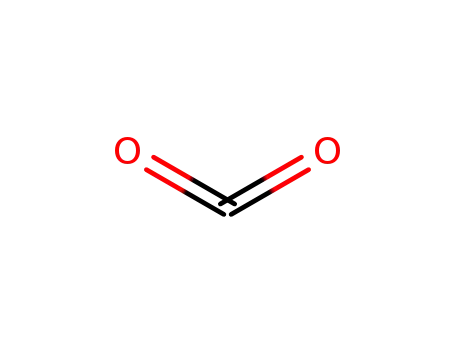
carbon dioxide

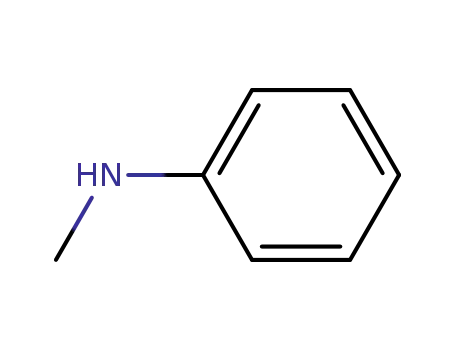
N-methylaniline
| Conditions | Yield |
|---|---|
|
With
lithium chloride;
In
1,4-dioxane;
at 25 ℃;
Rate constant;
Mechanism;
Thermodynamic data;
other N-alkyl-N-phenyl-varbamates, var. additives, var. temp.; deuterium kinetic isotope effect; ΔGact., var. LiCl conc.;
|
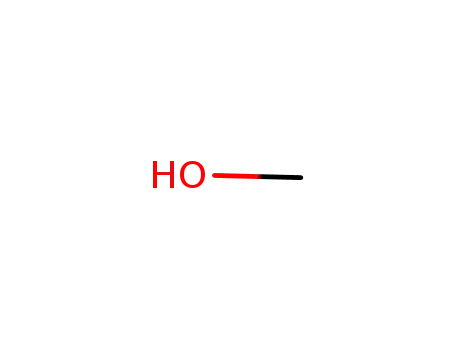
methanol
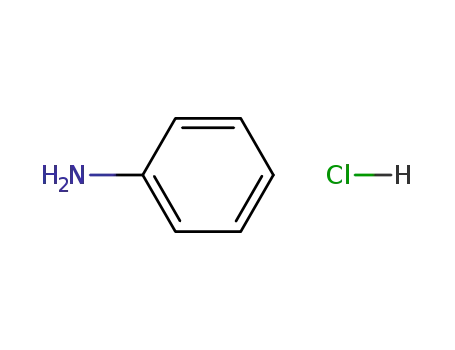
aniline hydrochloride
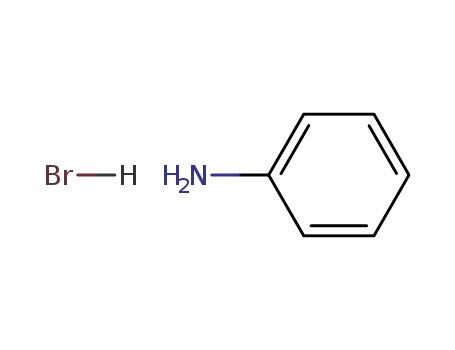
aniline hydrobromide
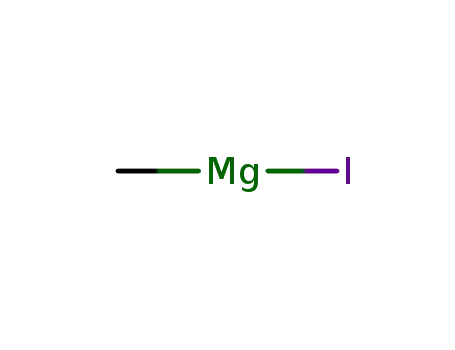
methyl magnesium iodide
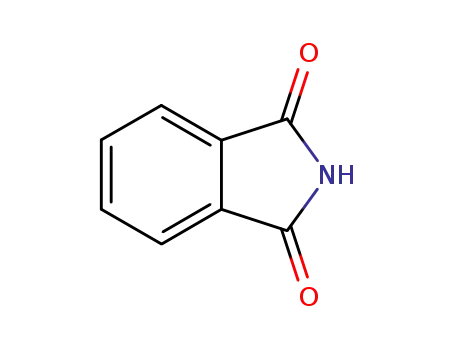
phthalimide
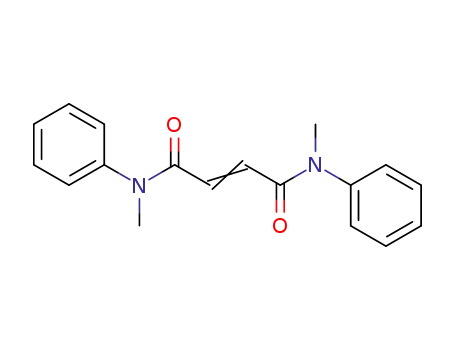
N,N'-dimethyl-N,N'-diphenyl-fumaramide
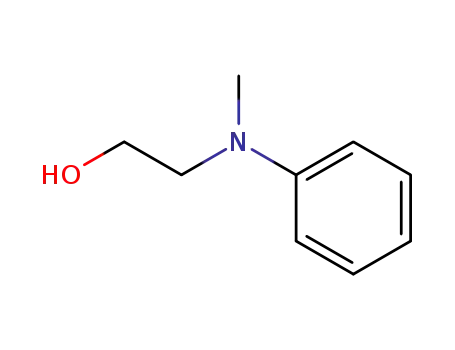
N-(2-hydroxyethyl)-N-methylaminobenzene
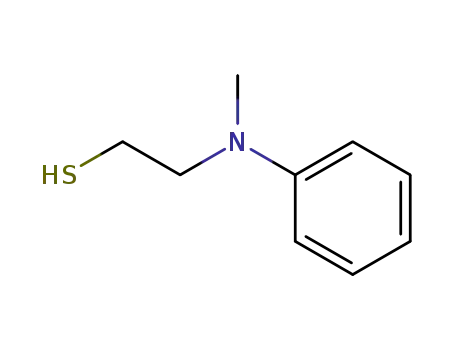
N-methyl-N-mercaptoethylaniline