
Antioxidant BHT 264
CAS:128-37-0
Purity:99%
Contact Now
We will contact you as soon as possible
Your Location:Home >Products >Industrial Chemicals >7440-70-2

Product Details
|
Physical properties |
Bright, silvery-white metal; face-centered cubic crystal structure (α = 0.5582 nm) at ordinary temperatures, transforming to body-centered cubic form (α = 0.4407) at 430°C; density 1.54 g/cm3 at 20°C; hardness 2 Mohs, 17 Brinnel (500 kg load); melts at 851°C; vaporizes at 1,482°C; electrical resistivity 3.43 and 4.60 microhm-cm at 0° and 20°C, respectively; modulus of elasticity 3-4x106 psi; mass magnetic susceptibility +1.10x10-6 cgs; surface tension 255 dynes/cm; brick-red color when introduced to flame (flame test); standard reduction potential E° = -2.87V. |
|
Production Methods |
Calcium may be obtained by electrolytic or thermal reduction of its salts.Electrolytic reduction involves electrolysis of partially molten calcium chloride at 780° to 800°C in a graphite lined steel vessel. The method requires precise control of temperature and current. The solid deposit of metal produced may contain entrapped salt and impurities such as chlorine and nitrogen. It is re-melted to reduce impurity levels.Currently, thermal reduction processes have replaced the electrolysis method. The starting material in these methods is limestone, which is calcined to produce calcium oxide. The latter is ground, mixed and compacted with aluminum, and reduced at temperatures between 1,000° to 1,200°C under vacuum. Calcium vapors formed in low yield under such thermodynamic conditions are transferred from the reactor and condensed in cool zones, thus shifting the equilibrium to allow formation of more calcium vapors. The reactions are as follows:4Ca + 2Al → CaO?Al2O3 + 3Ca (vapor)6Ca + 2Al → 3CaO?Al2O3 + 3Ca (vapor). |
|
Definition |
Alkaline-earth element of atomic number 20, group IIA of the periodic table. Aw 40.08. Valence 2. Six stable isotopes. |
|
General Description |
A silvery, soft metal that turns grayish white on exposure to air. Used in metallurgy. |
|
Air & Water Reactions |
Pyrophoric ignites in air when finely divided, then burns with crimson flame [Merck 11th ed. 1989]. Calcium rapidly decomposes in water, the heat of reaction is sufficient that hydrolysis released hydrogen may ignite [Lab. Gov. Chemist 1966]. |
|
Reactivity Profile |
Boron trifluoride reacts with incandescence when heated with alkali metals or alkaline earth metals except magnesium [Merck 11th ed. 1989]. Calcium reacts violently with acids [Lab. Govt. Chemist 1965]. Finely divided Calcium burns spontaneously in chlorine at elevated temperatures [Mellor 3:637, 638, 651 1946-47]. Finely divided or massive Calcium burns spontaneously in fluorine at ordinary temperatures. Calcium is incompatible with metal oxides, alkali metal hydroxides, chlorine fluorides, dinitrogen tetraoxide, and sulfur(with sulfur reacts explosively when ignited) [Bretherick, 5th Ed., 1995]. |
|
Hazard |
Evolves hydrogen with moisture. Flammable in finely divided state. Fire and explosion hazard when heated or on contact with strong oxidizing agents. |
|
Health Hazard |
Calcium is an essential nutrient for plants and animals, essential for bone, nervous system, and cell development. Recommended daily intakes for adults are between 800 and 1200 mg/day. Most of this is obtained in food; drinking water typically accounts for 50–300 mg/day, depending on the water hardness and assuming inges- tion of 2 L/day. Calcium in food and water is essentially nontoxic. A number of stud- ies suggest that water hardness protects against cardiovascular disease. One possible adverse effect from ingestion of high concentrations of calcium for long periods of time may be a greater risk of kidney stones. The presence of calcium in water decreases the toxicity of many metals to aquatic life. Stream standards for these met- als are expressed as a function of hardness and pH. Thus, the presence of calcium in water is beneficial and no limits on calcium have been established for protection of human or aquatic health. |
|
Fire Hazard |
Produce flammable gases on contact with water. May ignite on contact with water or moist air. Some react vigorously or explosively on contact with water. May be ignited by heat, sparks or flames. May re-ignite after fire is extinguished. Some are transported in highly flammable liquids. Runoff may create fire or explosion hazard. |
|
Mechanism of action |
About 48% of serum calcium is ionic, ca 46% is bound to blood proteins, the rest is present as diffusible complexes, e.g., of citrate. The calcium ion level must be maintained within definite limits. Bones act as a reservoir of certain ions, in particular Ca2+ and PO43-, which readily exchange between bones and blood. Bone structure comprises a strong organic matrix combined with an inorganic phase which is principally hydroxyapatite, 3Ca3(PO4)2·Ca(OH)2. Bones contain two forms of hydroxyapatite. The less soluble crystalline form contributes to the rigidity of the structure. The crystals are quite stable, but because of the small size present a very large surface area available for rapid exchange of ions and molecules with other tissues. There is also a more soluble intercrystalline fraction. Bone salts also contain small amounts of magnesium, sodium, carbonate, citrate, chloride, and fluoride. Osteoporosis is reported to result when bone resorption is relatively faster than bone formation. The calcium ion, necessary for blood-clot formation, stimulates release of bloodclotting factors from platelets. |
|
Potential Exposure |
Calcium is used as a raw material for aluminum, copper, and lead alloys. |
|
Carcinogenicity |
No studies on the carcinogenicity of elemental calcium were noted. The carcinogenicity of calcium chromate is attributed solely to intracellular soluble chromium. |
|
Purification Methods |
Clean the metal by washing it with ether to remove adhering paraffin, file the surface in an argon-filled glove box, and wash it with ethanol containing 2% of conc HCl. Then wash it with dry ethanol, dry it in a vacuum and store it under pure argon [Addison et al. J Chem Soc 3868 1962]. |
|
Incompatibilities |
Forms hydrogen gas on contact with air; finely divided material or dust may ignite spontaneously. A strong reducing agent; reacts violently with water, acids, strong oxidizers (such as chlorine, bromine, and fluorine), alkaline carbonates, dinitrogen tetroxide; halogenated hydrocarbons; lead chloride, halogens, alkaline hydroxides, oxygen, silicon, sulfur, chlorine, fluorine, chlorine trifluoride, and many other substances. Reacts with water to produce flammable hydrogen gas |
InChI:InChI=1/Ca
Isotypic imidonitridophosphates MH4P6N12...
A study is presented of the reaction Ca(...
The science of molten salt electrochemis...
Hydrogen release from Al-based complex h...
In this paper, the influences of microwa...
Stimulated by the discovery of the iron ...
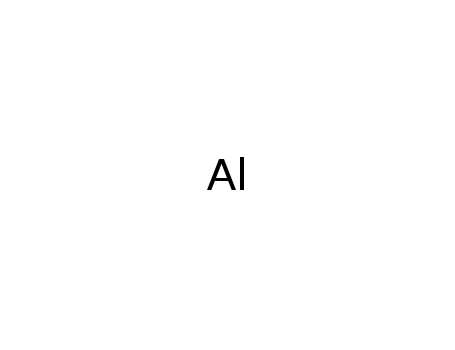
aluminium

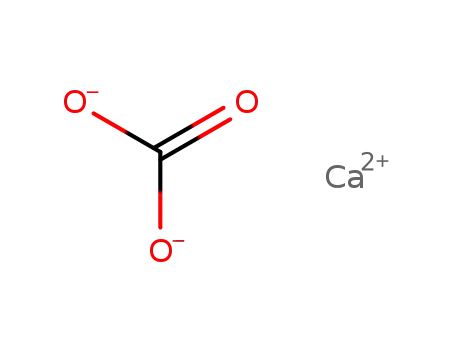
calcium carbonate

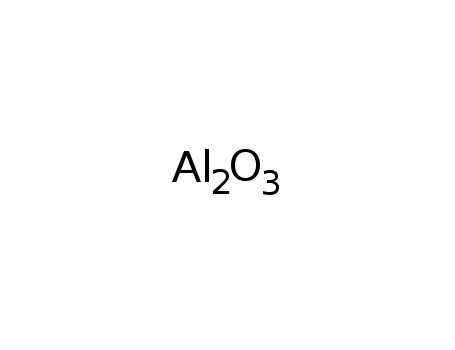
aluminum oxide


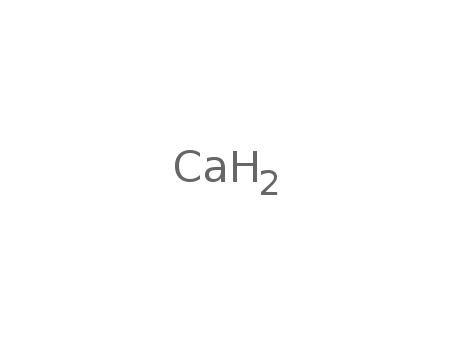
calcium
| Conditions | Yield |
|---|---|
|
In
neat (no solvent);
byproducts: C; heating equal amounts of carbonate and Al powder at red heat;; powdery product mixture obtained;;
|
|
|
In
neat (no solvent);
byproducts: C; heating equal amounts of carbonate and Al powder at red heat;; powdery product mixture obtained;;
|
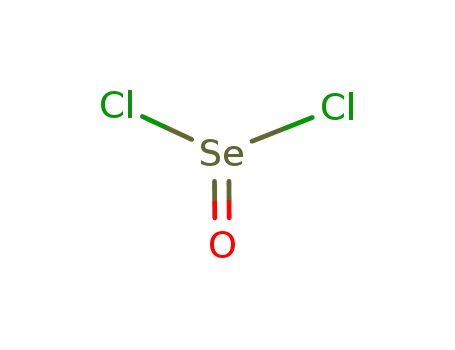
selenium oxychloride

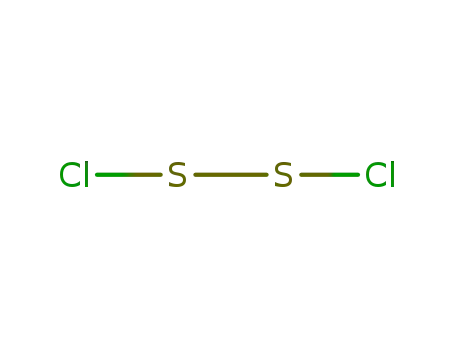
disulfur dichloride


calcium
| Conditions | Yield |
|---|---|
|
With
sulfur;
byproducts: SO2; heating; no reaction in cold SeOCl2;;
|
|
|
With
S;
byproducts: SO2; heating; no reaction in cold SeOCl2;;
|
sulfuric acid

aluminum oxide
calcium chloride
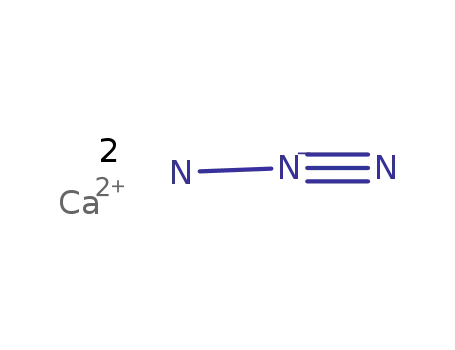
calcium azide
chromium

sodium
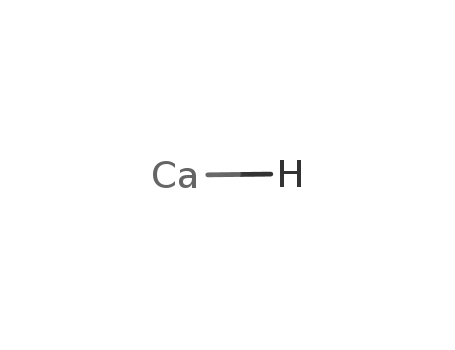
calcium monohydride
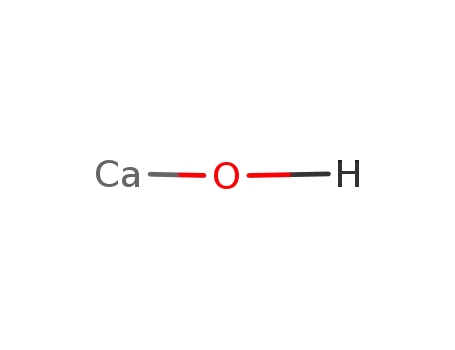
CaOH