
Antioxidant BHT 264
CAS:128-37-0
Purity:99%
Contact Now
We will contact you as soon as possible
Your Location:Home >Products >Intermediates >961-29-5

Product Details
|
Biochem/physiol Actions |
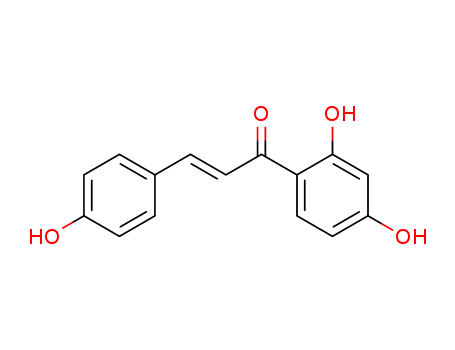
Isoliquiritigenin is a soluble guanylyl cyclase activator and possesses antitumor activity. It also possesses antioxidant, antiplatelet and estrogenic properties. |
|
Anticancer Research |
It is extracted from Dipteryx odorata seeds, found to be active in induction of quininereductase, and thus prevents chemical carcinogenesis. In a dose- and time-dependentmanner, it significantly inhibits the proliferation of prostate cancer cells,and this isoliquiritigenin-induced cell cycle arrest and antiproliferative effects maybe manifested by growth arrest- and DNA damage-inducible gene 153 (GADD153).It induces apoptosis in prostate cancer cells through mitochondrial apoptosispathway in which mitochondrial membrane potential is disrupted, cytochrome-cand Smac/Diablo are released, and caspase-9 is activated. It is reported to enhancethe expression of universal inhibitor of cyclin-dependent kinases, p21CIP1/WAF1, in adose- and time-dependent manner in A549 human lung cancer cells (Kanazawaet al. 2003; Ii et al. 2004; Balunas and Kinghorn 2005; Jung et al. 2006). |
|
General Description |
Isoliquiritigenin is an aromatic ketone and belongs to the chalcone group of compound. It is derived from licorice and is a component in medicine and food. |
InChI:InChI=1/C15H12O4/c16-11-4-1-10(2-5-11)3-8-14(18)13-7-6-12(17)9-15(13)19/h1-9,16-17,19H/b8-3+
Screening a natural product library of 8...
Glutamate-induced neurotoxicity is chara...
Bone diseases may not be imminently life...
Herein, we demonstrate that butein (1) c...
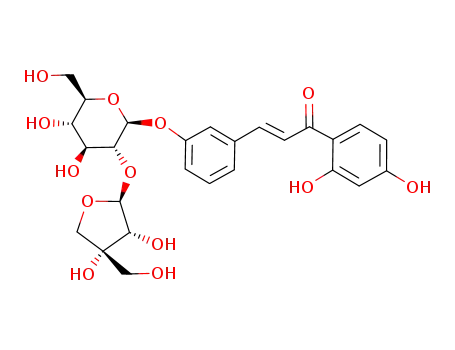
Neolicurosid

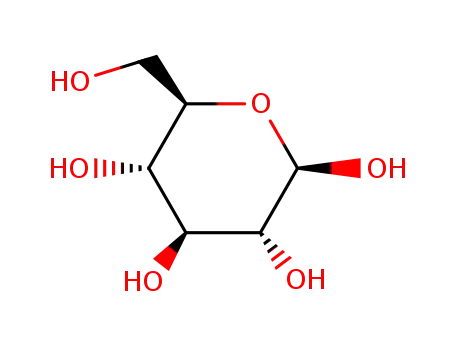
β-D-glucose

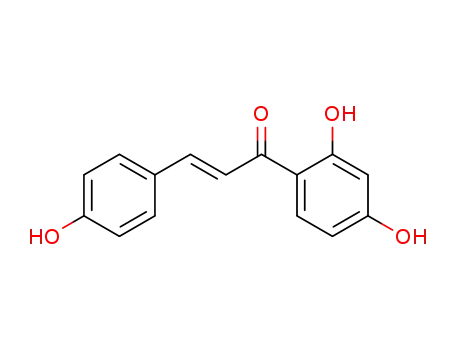
isoliquirtigenin

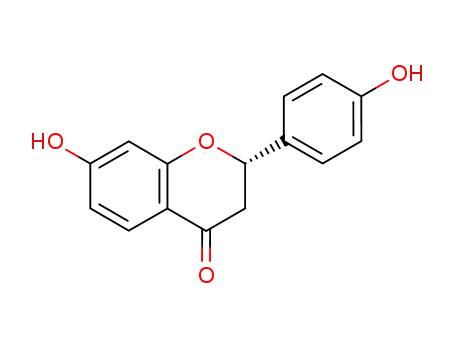
liquiritigenin

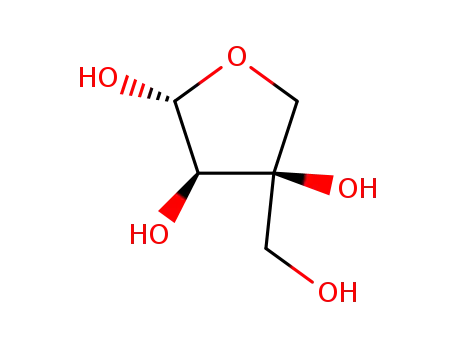
3-C-(hydroxymethyl)-β-D-erythrofuranose
| Conditions | Yield |
|---|---|
|
With
hydrogenchloride;
In
methanol; water;
at 70 ℃;
for 2h;
|
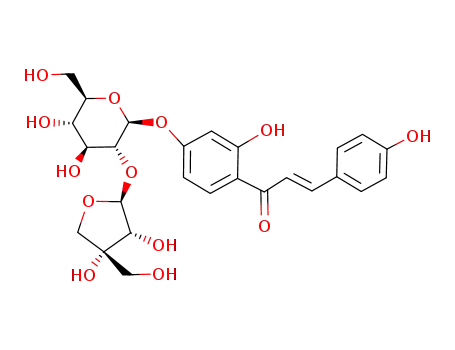
isoliquiritigenin-4'-O-β-D-apiosylglucoside


β-D-glucose


isoliquirtigenin


liquiritigenin


3-C-(hydroxymethyl)-β-D-erythrofuranose
| Conditions | Yield |
|---|---|
|
With
hydrogenchloride;
In
methanol; water;
at 70 ℃;
for 2h;
|
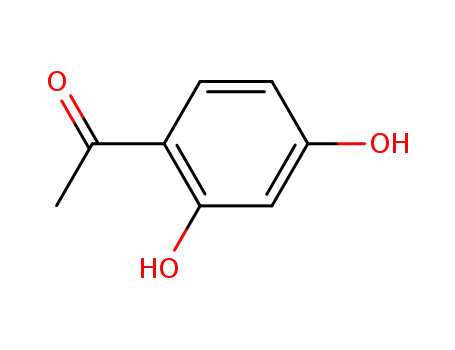
2',4'-dihydroxy-4-acetophenone
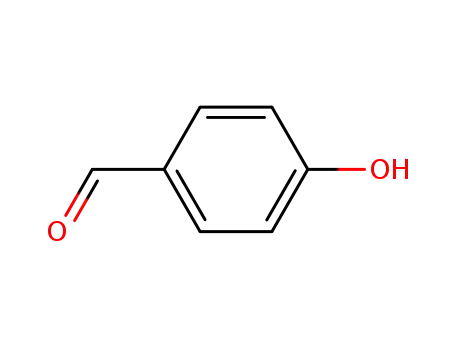
4-hydroxy-benzaldehyde
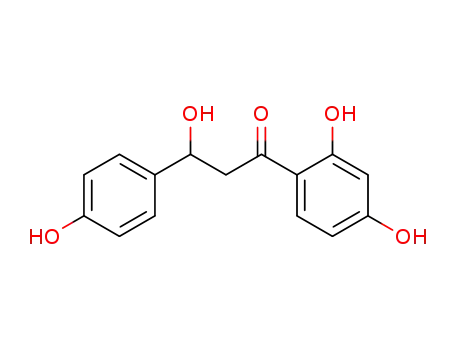
1-(2,4-dihydroxyphenyl)-3-hydroxy-3-(4'-hydroxyphenyl)-1-propanone
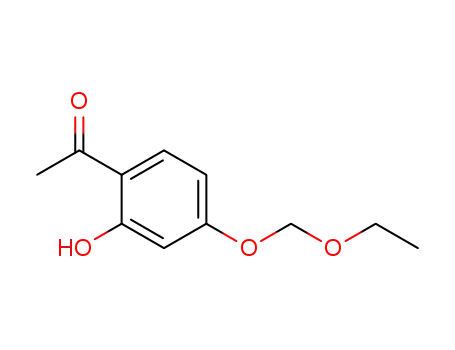
2'-Hydroxy-4'-(aethoxy-methoxy)-acetophenon
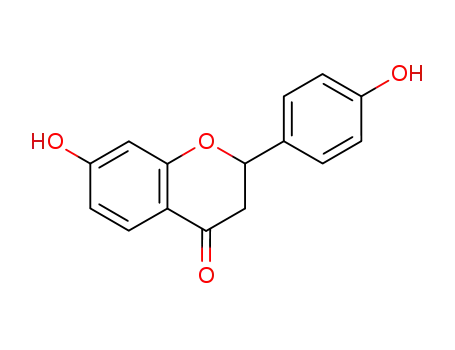
7,4'-dihydroxy-dihydroflavone
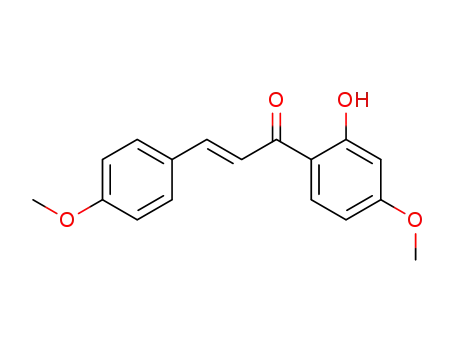
2'-hydroxy-4,4'-dimethoxychalcone
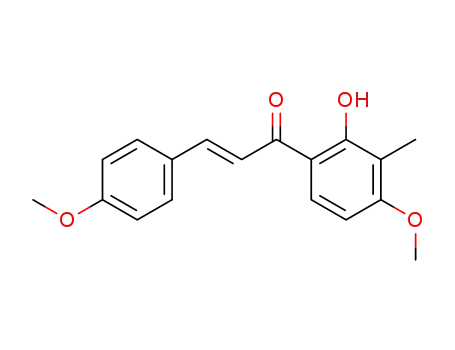
2'-hydroxy-4,4'-dimethoxy-3'-methyl-trans-chalcone
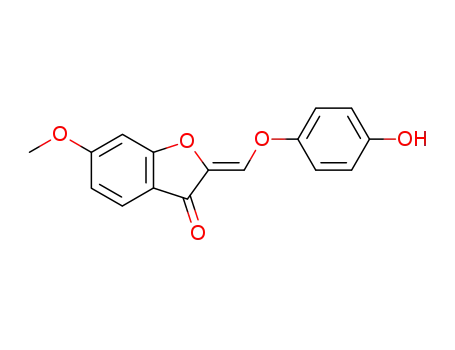
chalaurenol 6-O-methyl ether