
Antioxidant BHT 264
CAS:128-37-0
Purity:99%
Contact Now
We will contact you as soon as possible
Your Location:Home >Products >Pharmaceutical API >56824-22-7
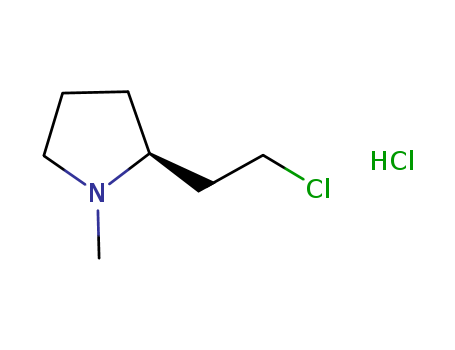

Product Details
InChI:InChI=1/C7H14ClN.ClH/c1-9-6-2-3-7(9)4-5-8;/h7H,2-6H2,1H3;1H
Neuronal nitric oxide synthase (nNOS) in...
The invention discloses a preparation me...
The first asymmetric synthesis of (R,R)-...
The first enantioselective synthesis of ...
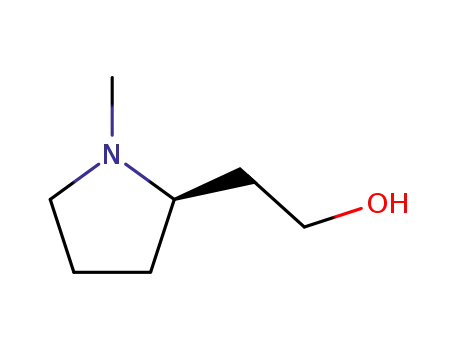
R(+)-1-methyl-2-(2-hydroxyethyl)pyrrolidine

(+)-(R)-2-(2-chloroethyl)-1-methylpyrrolidine hydrochloride
| Conditions | Yield |
|---|---|
|
With
thionyl chloride;
In
chloroform;
at 0 ℃;
for 1h;
Reflux;
|
100% |
|
R(+)-1-methyl-2-(2-hydroxyethyl)pyrrolidine;
With
hydrogenchloride;
In
chloroform;
for 0.5h;
With
thionyl chloride;
In
chloroform;
for 2h;
Reflux;
|
77% |
|
With
thionyl chloride;
In
chloroform;
at 0 ℃;
Reflux;
|
|
|
With
thionyl chloride;
In
dichloromethane;
at 0 - 25 ℃;
for 2h;
|
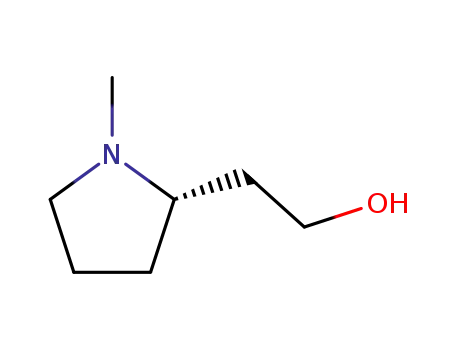
(-)-2-((S)-1-methylpyrrolidin-2-yl)ethanol

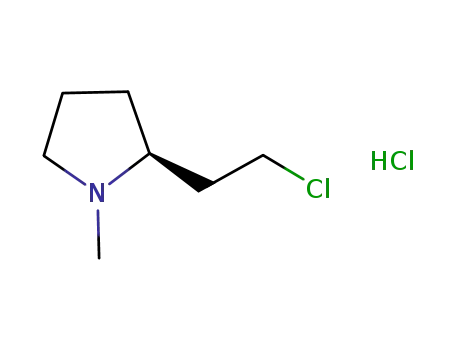
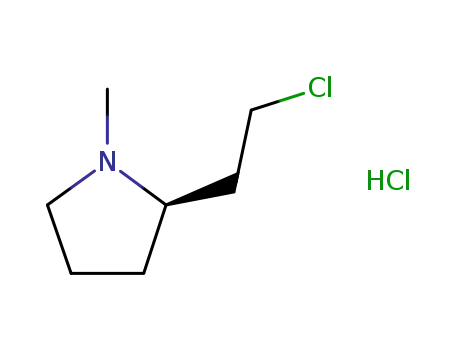
(-)-(S)-2-(2-chloroethyl)-1-methylpyrrolidine hydrochloride
| Conditions | Yield |
|---|---|
|
With
thionyl chloride;
In
chloroform;
at 0 ℃;
Reflux;
|
92% |

R(+)-1-methyl-2-(2-hydroxyethyl)pyrrolidine
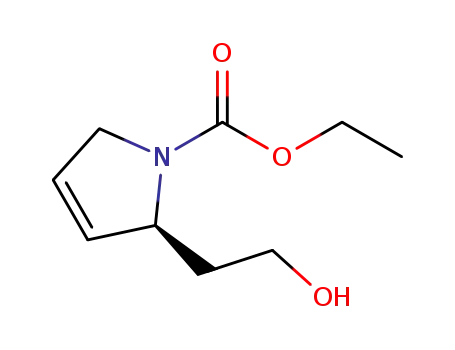
(S)-ethyl 2-(2-hydroxyethyl)-2,5-dihydro-1H-pyrrole-1-carboxylate
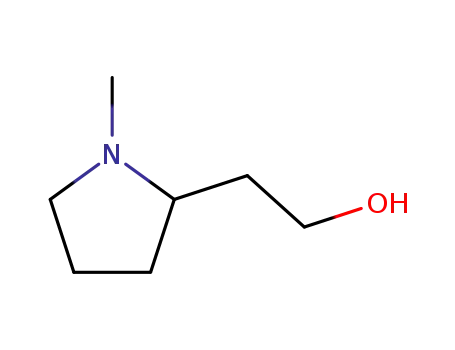
2-(1-methyl-2-pyrrolidine)ethanol

(-)-2-((S)-1-methylpyrrolidin-2-yl)ethanol