
Antioxidant BHT 264
CAS:128-37-0
Purity:99%
Contact Now
We will contact you as soon as possible
Your Location:Home >Products >Pharmaceutical API >52250-50-7

Product Details
InChI:InChI=1/C15H13N/c1-2-7-13(8-3-1)15-14-9-5-4-6-12(14)10-11-16-15/h1-9H,10-11H2
-
A route to the direct amidation of aroma...
Ten types of Tf2O/TTBP-mediated amide tr...
Tetraol-protected α-chiral allylboronate...
Superacid-promoted conversion of tetrahy...
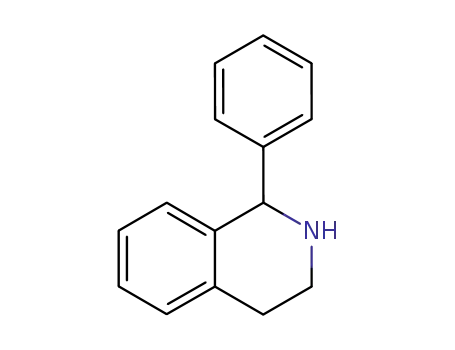
1-phenyl-1,2,3,4-tetrahydroisoquinoline

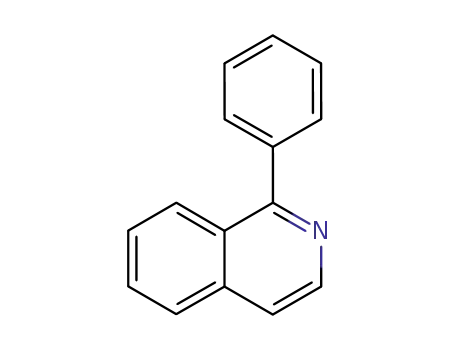
1-phenylisoquinoline

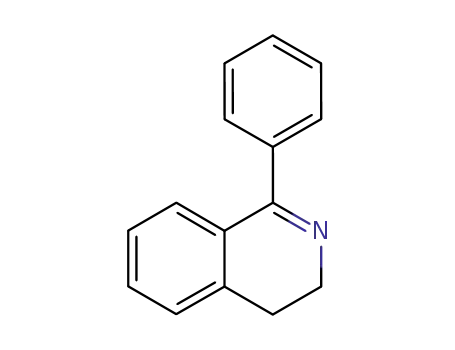
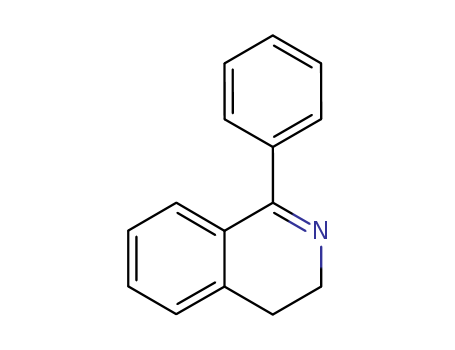
1-phenyl-3,4-dihydroisoquinoline
| Conditions | Yield |
|---|---|
|
With
potassium phosphate tribasic trihydrate; palladium on activated charcoal; oxygen;
In
acetonitrile;
at 60 ℃;
for 17h;
Solvent;
Temperature;
Reagent/catalyst;
|
86% |
|
With
air;
In
N,N-dimethyl-formamide;
at 100 ℃;
for 24h;
Temperature;
Solvent;
Concentration;
Schlenk technique;
Green chemistry;
|
83% |
|
With
sodium carbonate;
In
ethyl acetate;
at 120 ℃;
for 24h;
Overall yield = 99 %;
Sealed tube;
Green chemistry;
|
77% 22% |
|
With
1 wt % Rh0 photodeposited TiO2 nanoparticles;
In
isopropyl alcohol;
at 20 ℃;
for 48h;
Irradiation;
Inert atmosphere;
Sealed tube;
|
56% 32% |
|
With
oxygen; 4-amino-2,2,6,6-tetramethyl-1-piperidine-1-oxyl;
In
isopropyl alcohol;
at 20 ℃;
for 24h;
under 760.051 Torr;
Irradiation;
Sealed tube;
Sonication;
|
54% 33% |
|
With
potassium phosphate tribasic trihydrate; 5%-palladium/activated carbon;
In
acetonitrile;
at 60 ℃;
for 22h;
Reagent/catalyst;
Solvent;
Temperature;
chemoselective reaction;
Catalytic behavior;
Green chemistry;
|
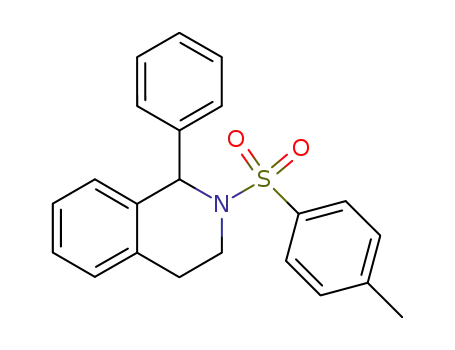
1-phenyl-2-tosyl-1,2,3,4-tetrahydroisoquinoline


1-phenylisoquinoline


1-phenyl-3,4-dihydroisoquinoline
| Conditions | Yield |
|---|---|
|
With
sodium hydroxide;
In
water; dimethyl sulfoxide;
at 125 ℃;
for 2h;
Inert atmosphere;
|
83% 9% |
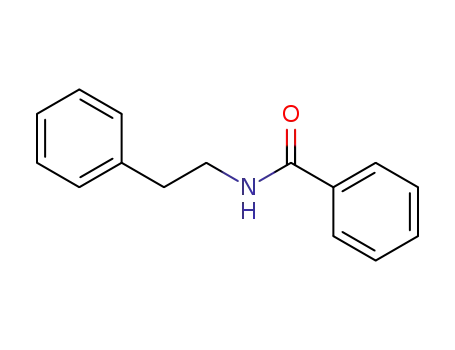
N-phenethylbenzamide
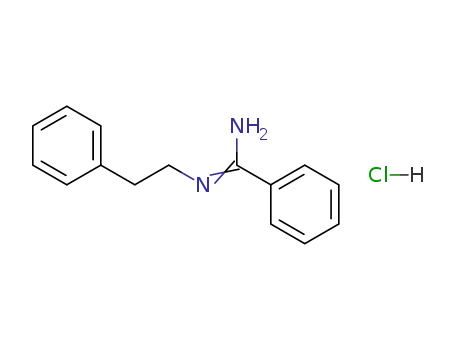
N-phenethyl-benzamidine; hydrochloride
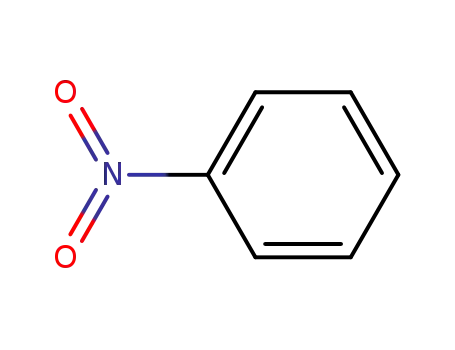
nitrobenzene
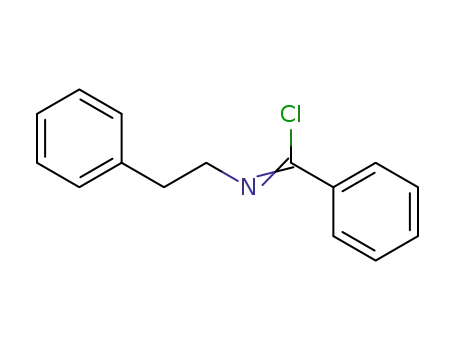
N-(2-phenethyl)benzimidoyl chloride
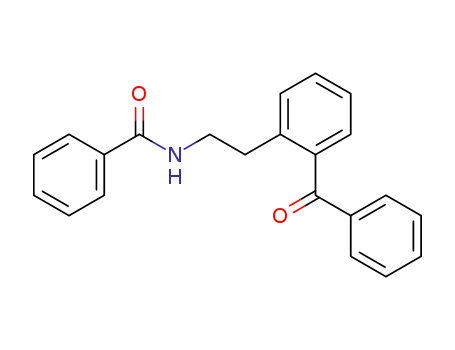
N-(2-benzoyl-phenethyl)-benzamide
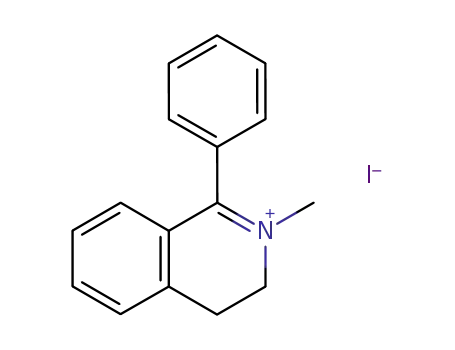
2-methyl-1-phenyl-3,4-dihydroisoquinolin-2-ium iodide

1-phenylisoquinoline
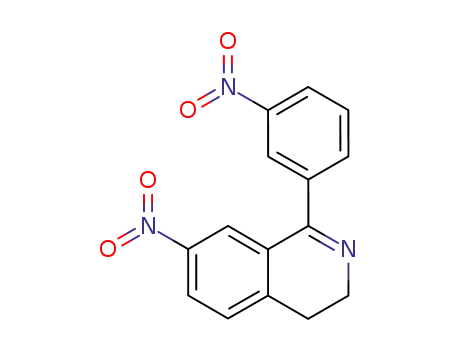
7-Nitro-1-(3-nitrophenyl)-3,4-dihydroisochinolin