
Antioxidant BHT 264
CAS:128-37-0
Purity:99%
Contact Now
We will contact you as soon as possible
Your Location:Home >Products >Pharmaceutical API >3562-99-0

Product Details
|
Manufacturing Process |
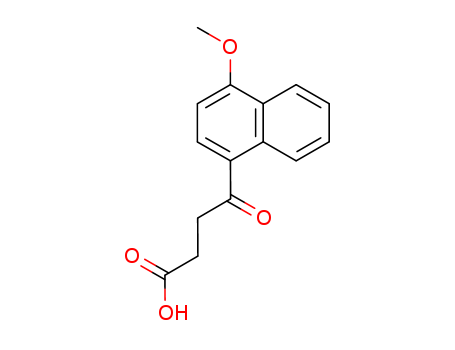
395 parts of (α-methoxynaphthalene and 265 parts of succinic anhydride are dissolved in 8,000 parts of dry benzene at room temperature. The resulting solution is stirred and 710 parts of anhydrous aluminum chloride are added over a period of twenty minutes. During the addition the temperature of the reaction mixture rises to about 60°C to 70°C. After the addition the reaction mixture is stirred for fifteen or twenty minutes at 60°C to 70°C and then refluxed for one hour. The hot reaction mixture is then poured onto a mixture of 5,000 parts of ice and 900 parts of concentrated hydrochloric acid. The benzene is removed by steam distillation and the hot aqueous residue is filtered to remove the insoluble β-(1-methoxy-4-naphthoyl)-propionic acid. The residue of the latter is dried and then dissolved in 16,000 parts of hot water containing 300 parts of sodium carbonate. The hot solution is treated with activated charcoal, filtered while hot, chilled and acidified. The residue of purified acid is collected on a filter, washed with water, and dried at 65°C. A yield of 552 parts of purified β-(1-methoxy)-4-naphthoyl)propionic acid, melting at 172°C to 173°C is obtained. |
|
Therapeutic Function |
Choleretic |
InChI:InChI=1/C14H12O3/c15-13(8-9-14(16)17)12-7-3-5-10-4-1-2-6-11(10)12/h1-7H,8-9H2,(H,16,17)
-
Alzheimer's disease (AD) is a severe age...
-
The invention discloses an improved synt...
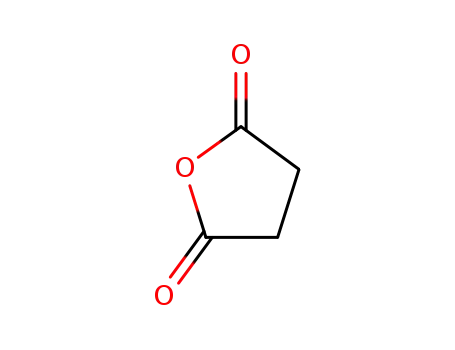
succinic acid anhydride

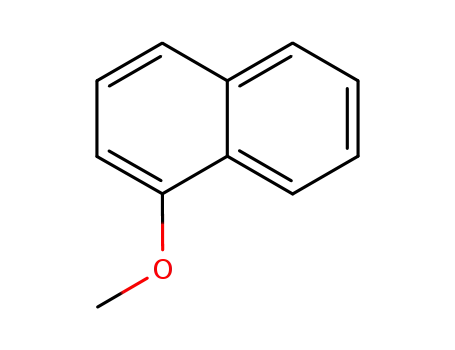
1-Methoxynaphthalene

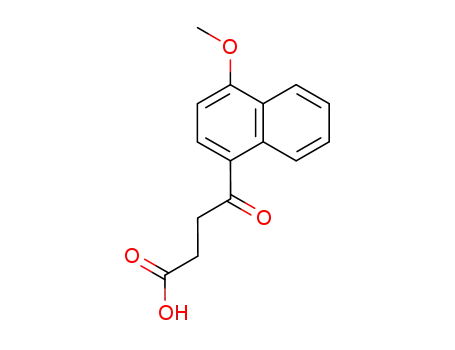
4-(4-methoxynaphthalen-1-yl)-4-oxobutanoic acid
| Conditions | Yield |
|---|---|
|
With
aluminum (III) chloride;
In
dichloromethane;
at 33 - 37 ℃;
for 6h;
|
86.4% |
|
With
carbon disulfide; aluminium trichloride;
|
|
|
With
aluminium trichloride; nitrobenzene;
|
|
|
With
aluminium trichloride;
|
|
|
With
aluminium trichloride; 1,1,2,2-tetrachloroethane;
|
|
|
With
aluminium trichloride;
In
nitrobenzene;
|
|
|
With
aluminium trichloride; nitrobenzene;
|
|
|
With
aluminum (III) chloride;
In
1,2-dichloro-ethane;
at 20 ℃;
for 4h;
|
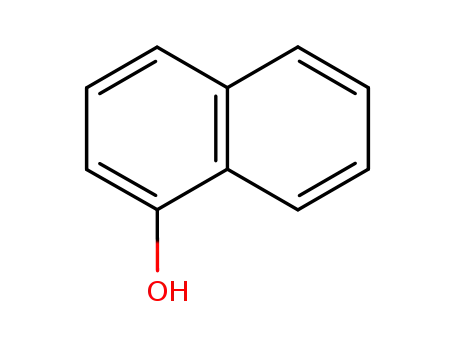
α-naphthol


4-(4-methoxynaphthalen-1-yl)-4-oxobutanoic acid
| Conditions | Yield |
|---|---|
|
Multi-step reaction with 2 steps
1: AlCl3; 1,1,2,2-tetrachloro-ethane
With
aluminium trichloride; 1,1,2,2-tetrachloroethane;
|

succinic acid anhydride

1-Methoxynaphthalene
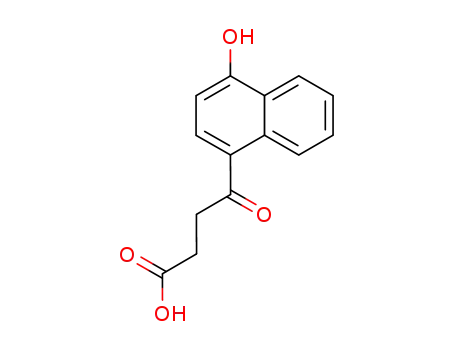
4-(4-hydroxy-[1]naphthyl)-4-oxo-butyric acid
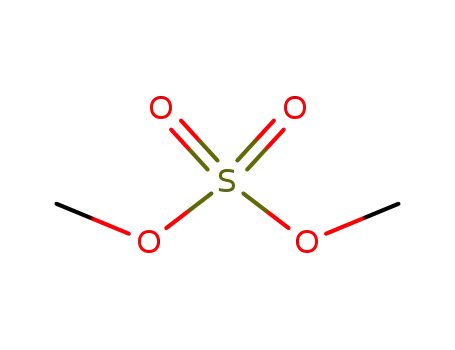
dimethyl sulfate
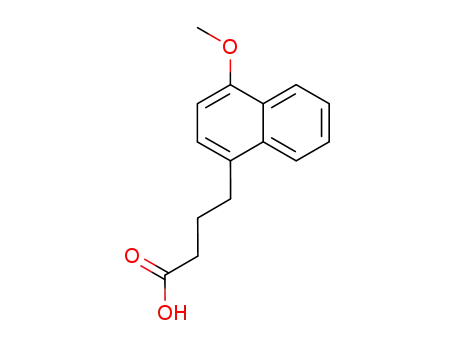
γ-(4-methoxy-1-naphthyl)butyric acid
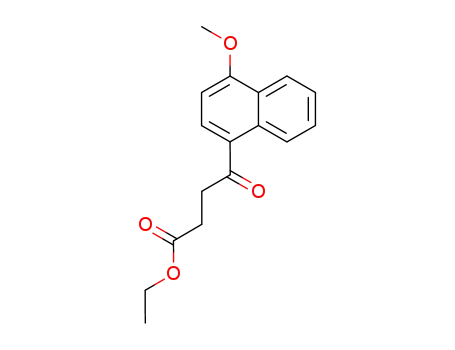
4-(4-methoxy-[1]naphthyl)-4-oxo-butyric acid ethyl ester
C37H44N4O5