
Antioxidant BHT 264
CAS:128-37-0
Purity:99%
Contact Now
We will contact you as soon as possible
Your Location:Home >Products >Industrial Chemicals >67-43-6
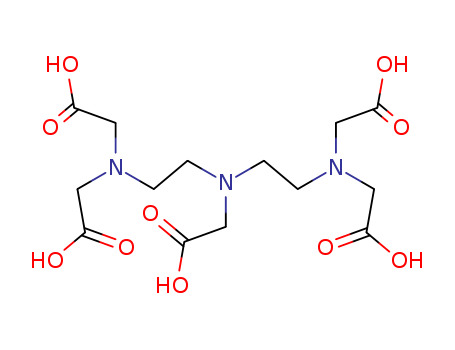

Product Details
|
Production Methods |
Pentetic acid is a pentaacetic acid triamine formed during the preparation of the amino carboxylic acid and its salt. |
|
Pharmaceutical Applications |
Pentetic acid is mainly used as a chelating agent in the preparation of imaging and contrast agents for radionuclide and magnetic resonance imaging.It is also used as a carrier excipient for neutron-capture isotopes in, for example, radiotherapy.Pentetic acid–isotope complexes have also been considered as model active substances in scintigraphic imaging studies.Pentetic acid has been used to chelate metal ions to reduce formation of reactive oxygen species during lyophilization. |
|
Safety Profile |
Moderately toxic by intraperitoneal route. When heated to decomposition it emits toxic fumes of NOx. |
|
Safety |
Pentetic acid is used in intrathecal and intravenous injection preparations. The pure form of pentetic acid is moderately toxic by the intraperitoneal route. LD50 (mouse, IP): 0.54 g/kg LD50 (mouse, oral): 4.84 g/kg |
|
storage |
The activity of pentetic acid as a chelating agent may cause unwanted effects in formulations containing metal ions. The desired chelate may be displaced by other ions from the formulation. |
|
Purification Methods |
Crystallise DTPA from water. Dry under vacuum or at 110o. [Bielski & Thomas J Am Chem Soc 109 7761 1987, NMR: Wenzel et al. Anal Chem 54 615 1982, Beilstein 4 IV 2454.] |
|
Incompatibilities |
The activity of pentetic acid as a chelating agent may cause unwanted effects in formulations containing metal ions. The desired chelate may be displaced by other ions from the formulation. |
|
Regulatory Status |
Included in the FDA Inactive Ingredients Database (intrathecal and intravenous injections). Included in intravenous and intra-articular injections licensed in the UK. |
|
General Description |
Visit our Titration Center to learn more. |
InChI:InChI=1/C4H13N3.5C2H4O2/c5-1-3-7-4-2-6;5*1-2(3)4/h7H,1-6H2;5*1H3,(H,3,4)
Agents for dyeing and/or lightening kera...
This invention relates to biochemistry a...
This invention relates to a method of us...
There is disclosed a process for produci...
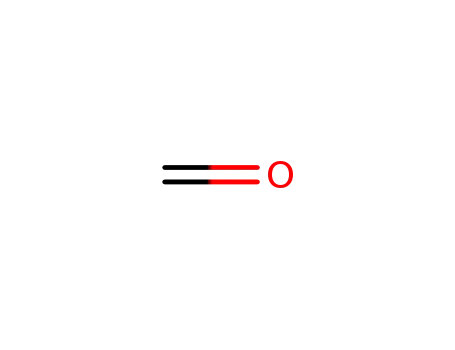
formaldehyd

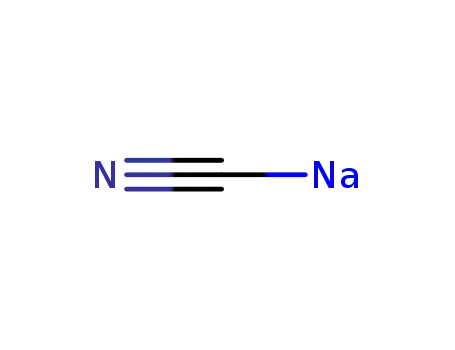
sodium cyanide

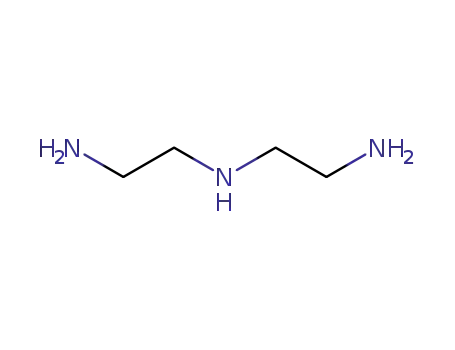
3-azapentane-1,5-diamine

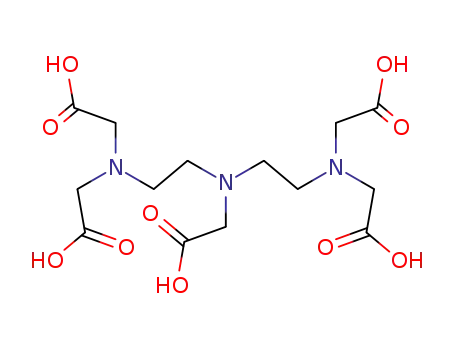
diethylenetriaminopentaacetic acid
| Conditions | Yield |
|---|---|
|
With
aqueous alkali;
|
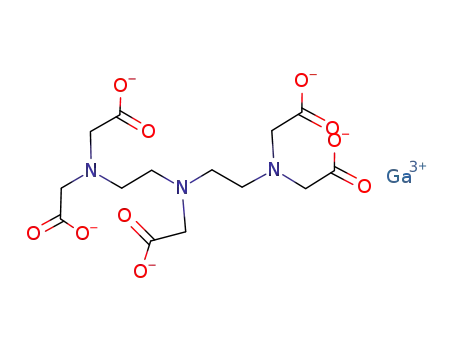
diethylenetriaminepentaacetic acid, Ga(III)-salt, dianion

2-(HO)-5-(SO3H)C6H3CHNC2H4NHC2H4NHC2H4NCHC6H3-2-(OH)-5-(SO3H)

Ga(N(CHC6H3-2-O-5-SO3))CH2NHC2H4NHC2H4NCH(C6H3-2-O-5-SO3)


diethylenetriaminopentaacetic acid
| Conditions | Yield |
|---|---|
|
In
water-d2;
competition was followed by H-NMR spectroscopy, at 25 ° C;; aq. soln. of known pH evapd. to dryness, residue dissolved in equal amt. of D2O, left until equilibrium was reached (5 weeks);
|
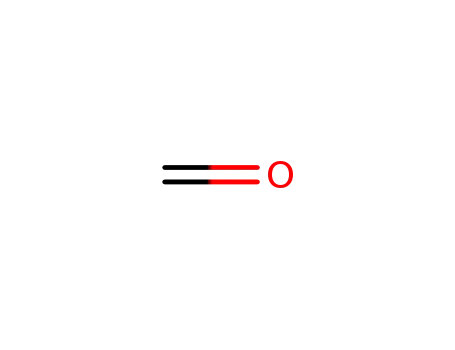
formaldehyd
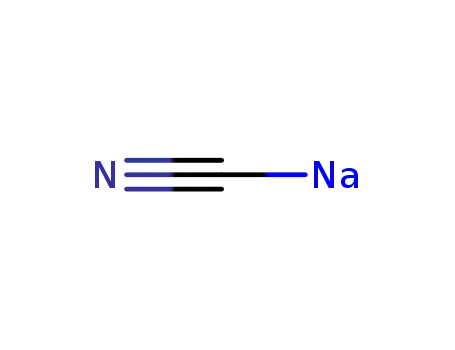
sodium cyanide

3-azapentane-1,5-diamine

diethylenetriaminepentaacetic acid, Ga(III)-salt, dianion
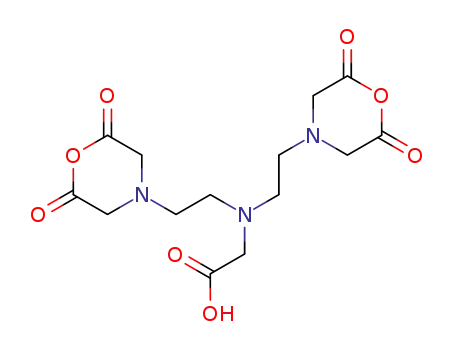
diethylenetriaminepentaacetic dianhydride
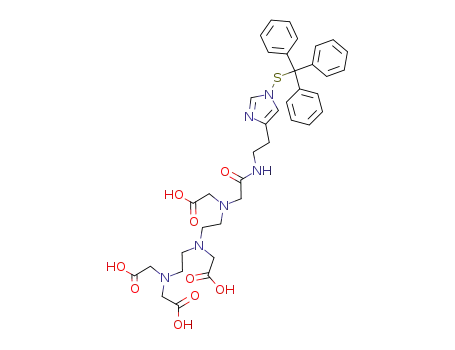
((2-{[2-(bis-carboxymethyl-amino)-ethyl]-carboxymethyl-amino}-ethyl)-{[2-(1-tritylsulfanyl-1H-imidazol-4-yl)-ethylcarbamoyl]-methyl}-amino)-acetic acid
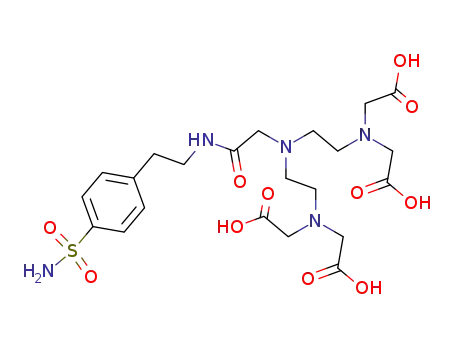
{[2-([2-(bis-carboxymethyl-amino)-ethyl]-{[2-(4-sulfamoyl-phenyl)-ethylcarbamoyl]-methyl}-amino)-ethyl]-carboxymethyl-amino}-acetic acid