
Antioxidant BHT 264
CAS:128-37-0
Purity:99%
Contact Now
We will contact you as soon as possible
Your Location:Home >Products >Industrial Chemicals >128-37-0

Product Details
|
description |
Butylated hydroxytoluene is a synthetic phenolic compound mainly used as an antioxidant and preservative in the food industry. It is used to prevent the lipid oxidation in oils and fat-containing foods.Butylated Hydroxytoluene?toxicity is generally considered as being low.Since Butylated Hydroxytoluene?is used in many near consumer products population wide exposure is expected. |
|
Application from Literature |
The applications of butylated hydroxytoluene (BHT) have been reported as following [1-9]: ? Butylated hydroxytoluene metabolites causing DNA strand breaks in cultured cells and DNA breaks between nucleosomes (a typical feature of apoptosis), which result in relieving inflammation. ? Inhibiting secretion, aggregation, and protein phosphorylation caused by protein kinase C activators at the process of the pre-incubation of aspirin-treated platelets. ? Inhibiting liver cancer formation induced by aflatoxin B1. ? As Michael receptor, butylated hydroxytoluene can react with uninucleophiles and proteins. ? Reaction of 2, 6-di-tert-butyl-4-methylphenol with fluorine (II) - benzophenone dianion complex. ? Food additive 2, 6-di-tert-butyl-4-methylphenol can promote acute lung toxicity and tumor growth in mice. ? Butylated hydroxytoluene can be used to prepare organoaluminum compound methylaluminum bis (2, 6-di-tert-butyl-4-alkylphenol oxide). |
|
Mammalian physiology |
Butylated Hydroxytoluene is a phenolic antioxidant. Butylated Hydroxytoluene can inhibit lipid peroxidation and cause lung injury in mice and promote tumor growth, which may be due to the metabolites of Butylated Hydroxytoluene, 6-tert-butyl-2-[2′-(2′-hydroxymethyl)-propyl]-4-Methylphenol. Butylated Hydroxytoluene metabolites have also been reported to cause DNA strand breaks in cultured cells and DNA breaks between nucleosomes (a typical feature of apoptosis). A single intraperitoneal injection of Butylated Hydroxytoluene (60mg/kg body weight) into rats caused a significant increase in nuclear DNA methyltransferase activity in the liver, kidney, heart, spleen, brain, and lung. |
|
Definition |
ChEBI: A member of the class of phenols that is 4-methylphenol substituted by tert-butyl groups at positions 2 and 6. |
|
Preparation |
Butylated Hydroxytoluene is produced commercially by the alkylation of para-cresol with isobutylene. Butylated Hydroxytoluene is also produced by several western European manufacturers, production/processing plants in Germany, France, the Netherlands, United Kingdom and Spain. |
|
Production Methods |
Prepared by the reaction of p-cresol with isobutene. |
|
General Description |
White crystalline solid. |
|
Air & Water Reactions |
Insoluble in water. |
|
Reactivity Profile |
Phenols, such as 2,6-Di-tert-butyl-4-methylphenol, do not behave as organic alcohols, as one might guess from the presence of a hydroxyl (-OH) group in their structure. Instead, they react as weak organic acids. Phenols and cresols are much weaker as acids than common carboxylic acids (phenol has Ka = 1.3 x 10^[-10]). These materials are incompatible with strong reducing substances such as hydrides, nitrides, alkali metals, and sulfides. Flammable gas (H2) is often generated, and the heat of the reaction may ignite the gas. Heat is also generated by the acid-base reaction between phenols and bases. Such heating may initiate polymerization of the organic compound. Phenols are sulfonated very readily (for example, by concentrated sulfuric acid at room temperature). The reactions generate heat. Phenols are also nitrated very rapidly, even by dilute nitric acid. Nitrated phenols often explode when heated. Many of them form metal salts that tend toward detonation by rather mild shock. May react with oxidizing materials. |
|
Health Hazard |
2,6-Di-tert-butyl-p-cresol or Butylated Hydroxytoluene is of relatively low acute toxicity in animals, and there is no evidence of either acute or chronic effects among exposed workers. |
|
Fire Hazard |
2,6-Di-tert-butyl-4-methylphenol is combustible. |
|
Flammability and Explosibility |
Nonflammable |
|
Pharmaceutical Applications |
Butylated hydroxytoluene is used as an antioxidant in cosmetics, foods, and pharmaceuticals. It is mainly used to delay or prevent the oxidative rancidity of fats and oils and to prevent loss of activity of oil-soluble vitamins. Butylated hydroxytoluene is also used at 0.5–1.0% w/w concentration in natural or synthetic rubber to provide enhanced color stability. Butylated hydroxytoluene has some antiviral activity and has been used therapeutically to treat herpes simplex labialis. |
|
Biochem/physiol Actions |
Butylated hydroxytoluene is a phenolic antioxidant. It has been shown to inhibit lipid peroxidation. It causes lung injury and promotes tumors in mice, but this may be due to a metabolite of Butylated Hydroxytoluene, 6-tert-butyl-2-[2′-(2′-hydroxymethyl)-propyl]-4-methylphenol. Metabolites of Butylated Hydroxytoluene have also been reported to induce DNA strand breaks and internucleosomal DNA fragmentation (a characteristic of apoptosis) in cultured cells. In rats, a single intraperitoneal injection of Butylated Hydroxytoluene (60 mg/kg body mass) results in a significant increase in nuclear DNA methyl transferase activity in the liver, kidneys, heart, spleen, brain and lungs. Incubation of alveolar macrophages with Butylated Hydroxytoluene?significantly reduced the level of TNF-α which may explain the mechanism by which this antioxidant reduces inflammation. Preincubation of aspirin-treated platelets with Butylated Hydroxytoluene inhibits the secretion, aggregation, and protein phosphorylation induced by protein kinase C activators. Butylated Hydroxytoluene was also found to inhibit the initiation of hepatocarcinogenesis by aflatoxin B1. |
|
Contact allergens |
This antioxidant is contained in food, adhesive glues, industrial oils, and greases, including cutting fluids. Sensitization seems very rare. |
|
Carcinogenicity |
The IARC has determined that there is limited evidence for the carcinogenicity of Butylated Hydroxytoluene?in experimental animals.Butylated Hydroxytoluene?has given primarily negative results in a large number of in vivo and in vitro genotoxic assays.No significant reproductive effects were observed in three-generation toxicity studies in mice administered up to 0.4% in the diet.6 The 2003 ACGIH threshold limit valuetime- weighted average (TLV-TWA) for 2,6-ditert- butyl-p-cresol is 2mg/m3. |
|
Environmental Fate |
The metabolites of Butylated Hydroxytoluene can bind to cellular macromolecules, such as proteins and DNA, and cause toxicity. |
|
Potential Exposure |
DBPC is used as a food and feed additive, flavor, and packaging material; as an antioxidant in human foods and animal feeds. It is also used as an antioxidant to sta- bilize petroleum fuels, rubber and vinyl plastics. |
|
Safety Profile |
Poison by intraperitoneal andintravenous routes. Moderately toxic by ingestion. Anexperimental teratogen. Other experimental reproductiveeffects. A human skin irritant. A skin and eye irritant.Questionable carcinogen with experimental carcinogenicand. |
|
Safety |
Butylated hydroxytoluene is readily absorbed from the gastrointestinal tract and is metabolized and excreted in the urine mainly as glucuronide conjugates of oxidation products. Although there have been some isolated reports of adverse skin reactions, butylated hydroxytoluene is generally regarded as nonirritant and nonsensitizing at the levels employed as an antioxidant. The WHO has set a temporary estimated acceptable daily intake for butylated hydroxytoluene at up to 125 μg/kg body-weight. Ingestion of 4 g of butylated hydroxytoluene, although causing severe nausea and vomiting, has been reported to be nonfatal. LD50 (guinea pig, oral): 10.7 g/kg LD50 (mouse, IP): 0.14 g/kg LD50 (mouse, IV): 0.18 g/kg LD50 (mouse, oral): 0.65 g/kg LD50 (rat, oral): 0.89 g/kg |
|
storage |
Exposure to light, moisture, and heat causes discoloration and a loss of activity. Butylated hydroxytoluene should be stored in a wellclosed container, protected from light, in a cool, dry place. |
|
Shipping |
UN2811 Toxic solids, organic, n.o.s., Hazard Class: 6.1; Labels: 6.1-Poisonous materials, Technical Name Required. |
|
Purification Methods |
Dissolve Butylated Hydroxytoluene in n-hexane at room temperature, then cool with rapid stirring, to -60o. The precipitate is separated, redissolved in hexane, and the process is repeated until the mother liquor is no longer coloured. The final product is stored under N2 at 0o [Blanchard J Am Chem Soc 82 2014 1960]. It has also been recrystallised from EtOH, MeOH, *benzene, n-hexane, methylcyclohexane or pet ether (b 60-80o), and is dried in a vacuum. [Beilstein 6 IV 3511.] |
|
Toxicity evaluation |
Butylated Hydroxytoluene is a white crystalline solid. It is insoluble in water and alkalies; but soluble in most common organic solvents such as alcohol and ether. Its melting point is 70°C, boiling point is 265°C, flash point is 127°C, and specific gravity is 1.048 at 20°C. |
|
Incompatibilities |
Butylated hydroxytoluene is phenolic and undergoes reactions characteristic of phenols. It is incompatible with strong oxidizing agents such as peroxides and permanganates. Contact with oxidizing agents may cause spontaneous combustion. Iron salts cause discoloration with loss of activity. Heating with catalytic amounts of acids causes rapid decomposition with the release of the flammable gas isobutene. |
|
Regulatory Status |
GRAS listed. Accepted as a food additive in Europe. Included in the FDA Inactive Ingredients Database (IM and IV injections, nasal sprays, oral capsules and tablets, rectal, topical, and vaginal preparations). Included in nonparenteral medicines licensed in the UK. Included in the Canadian List of Acceptable Non-medicinal Ingredients. |
InChI:InChI=1/C15H24O/c1-10-8-11(14(2,3)4)13(16)12(9-10)15(5,6)7/h8-9,16H,1-7H3
Decay of the 2,6-di-tert-butyl-4-methylp...
Novel and environmentally benign Bronste...
The equilibrium constants, K1, for the r...
-
Degradation products of terbutol (2,6-di...
In this work, tertiary butylation of p-c...
-
The present study describes the synthesi...
tert-Butyl-substituted phenols have been...
The results of 1H NMR and quantum chemic...
The extra 'stabilization' of the 2,2,4,6...
-
A kinetic scheme is proposed for the rea...
-
The reaction of 2> with the compounds Ph...
We report a one-step procedure to direct...
The microwave-accelerated Claisen rearra...
The invention belongs to the technical f...
Acid Orange 7

ethylbenzene

(1S)-camphor

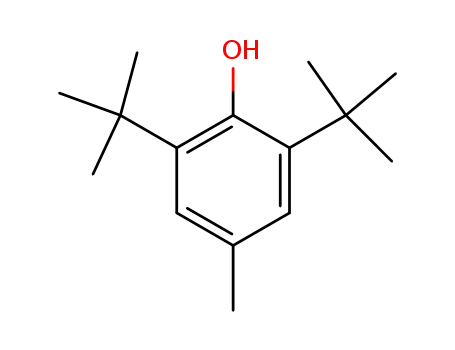
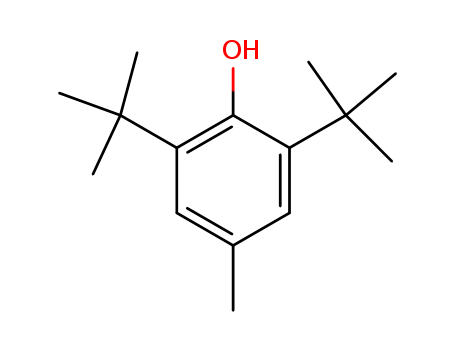
2,6-di-tert-butyl-4-methyl-phenol

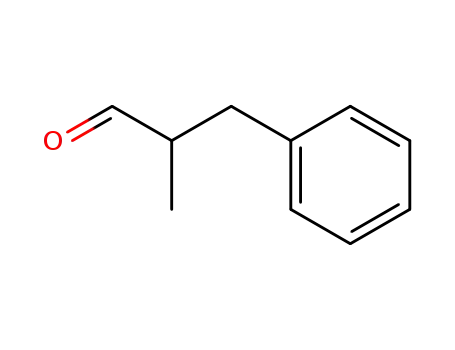
2-benzylpropionaldehyde

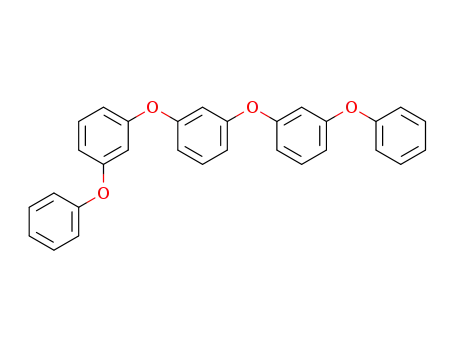
1,3-bis(3-phenoxyphenoxy)benzene

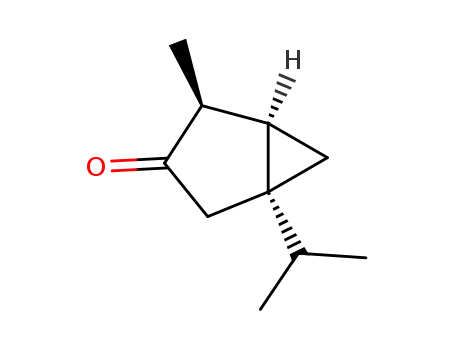
(+)-isothujone

acetic acid

acrylic acid

limonene.
| Conditions | Yield |
|---|---|
|
With
zinc(II) oxide;
Reagent/catalyst;
Concentration;
pH-value;
Sonication;
Darkness;
|
![3,3',5,5'-tetra-tert-butyl-1,1'-dimethyl-[1,1'-bi(cyclohexane)]-2,2',5,5'-tetraene-4,4'-dione](/upload/2025/1/a3c38b61-5e7a-4de7-bda2-9aaf0e9bb1f9.png)
3,3',5,5'-tetra-tert-butyl-1,1'-dimethyl-[1,1'-bi(cyclohexane)]-2,2',5,5'-tetraene-4,4'-dione

2,6-di-tert-butyl-4-methoxymethylene-phenol


2,6-di-tert-butyl-4-methyl-phenol

2,6-di-tert-butyl-4-methylene-2,5-cyclohexadien-1-one

4-methoxy-4-methyl-2,6-ditert-butylcyclohexa-2,5-dienone
| Conditions | Yield |
|---|---|
|
With
methanol; trifluorormethanesulfonic acid;
at 30 ℃;
for 0.35h;
|
2,6-di-tert-butylphenol
4,4'-Methylenebis(2,6-di-tert-butylphenol)
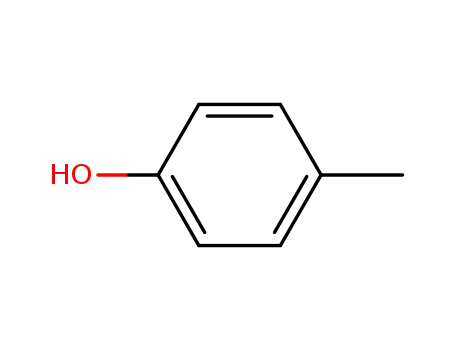
p-cresol
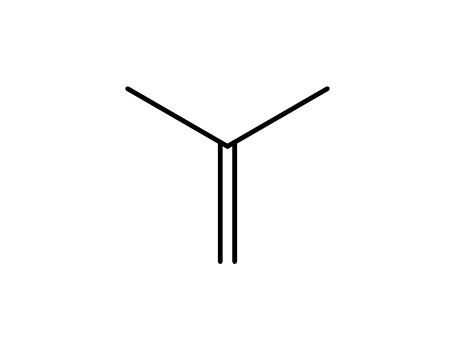
isobutene

4-methoxy-4-methyl-2,6-ditert-butylcyclohexa-2,5-dienone
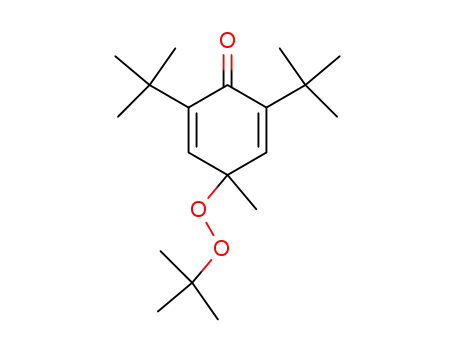
2,6-di-tert-butyl-4-(tert-butylperoxy)-4-methylcyclohexa-2,5-dien-1-one
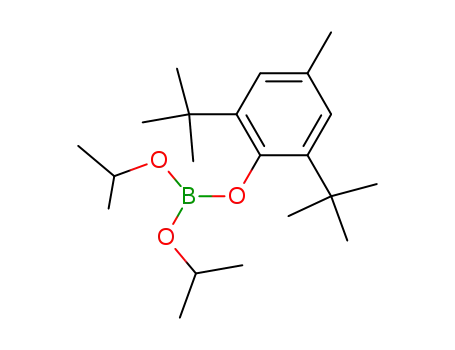
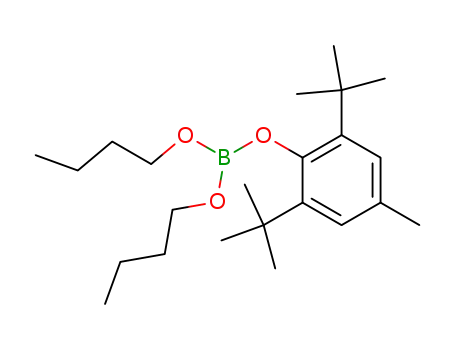
2,6-Di-t-butyl-p-kresyl-diisopropylborat
(C4H9O)2B(O-C6H2-2.6-(t-C4H9)2CH3)