
Antioxidant BHT 264
CAS:128-37-0
Purity:99%
Contact Now
We will contact you as soon as possible
Your Location:Home >Products >Intermediates >156-38-7
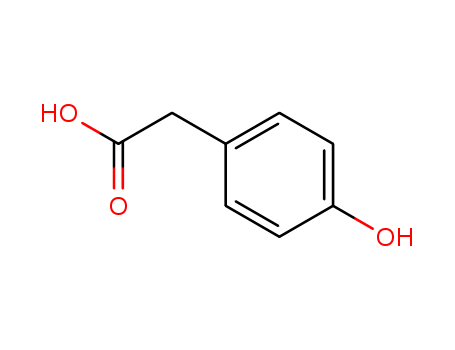

Product Details
|
Referrence |
Liu, Zhongyang, et al. "4-Hydroxyphenylacetic Acid Attenuated Inflammation and Edema via Suppressing HIF-1α in Seawater Aspiration-Induced Lung Injury in Rats." International Journal of Molecular Sciences 15.7(2014):12861-84. Ng, T.B.; Liu, F.; Lu, Y.; Cheng, C.H.; Wang, Z. Antioxidant activity of compounds from the medicinal herb Aster tataricus. Comp. Biochem. Physiol. 2003, 136, 109–115.? Du, L.; Mei, H.F.; Yin, X.; Xing, Y.Q. Delayed growth of glioma by a polysaccharide from Aster tataricus involve upregulation of Bax/Bcl-2 ratio, activation of caspase-3/8/9, and downregulation of the Akt. Tumour Biol. 2014, 35, 1819–1825. |
|
Preparation |
4-Hydroxyphenylacetic acid is synthesized by diazotization and hydrolysis of 4-aminophenylacetic acid. 4-aminophenylacetic acid and alkali solution are prepared into sodium salt, and then sulfuric acid is added. Cool to 0°C, control the temperature at 0-5°C, and add sodium nitrate solution dropwise, and the reaction is completed for 0.5h. The obtained diazonium was added dropwise to dilute sulfuric acid at 90-95°C for about 1 hour, and the reaction was continued for 1 hour. The reaction solution was decolorized and filtered, cooled and extracted with ethyl acetate, and the extract was recovered with ethyl acetate to obtain 4-Hydroxyphenylacetic acid. The yield is about 85%. |
|
Flammability and Explosibility |
Notclassified |
|
Purification Methods |
Crystallise the acid from water or Et2O/pet ether. The p-bromophenacyl ester has m 117o (from EtOH). [Beilstein 10 II 112, 10 III 430, 10 IV 543.] |
|
Definition |
ChEBI: 4-hydroxyphenylacetic acid is a monocarboxylic acid that is acetic acid in which one of the methyl hydrogens is substituted by a 4-hydroxyphenyl group. It has a role as a plant metabolite, a fungal metabolite, a human metabolite and a mouse metabolite. It is a monocarboxylic acid and a member of phenols. It derives from an acetic acid. It is a conjugate acid of a 4-hydroxyphenylacetate. |
InChI:InChI=1/C8H8O3/c9-7-3-1-6(2-4-7)5-8(10)11/h1-4,9H,5H2,(H,10,11)/p-1
Key Word Index - Taraxacum officinale; C...
-
Caged compounds are molecules that relea...
The p-hydroxyphenacyl group 1 is an effe...
Cell suspension cultures of Syringa vulg...
The oxidizing ability of peroxodisulfate...
-
-
A bioorthogonal 'catch and photorelease'...
1,2-Aryl migration of 1-halomethyl-2-(4-...
From the roots of Lactuca laciniata, six...
Two new germacranolides, ixerins H and I...
Biomass-derived hydroxycinnamates (mainl...
Aequorin consists of apoprotein (apoAequ...
The green and sustainable synthesis of c...
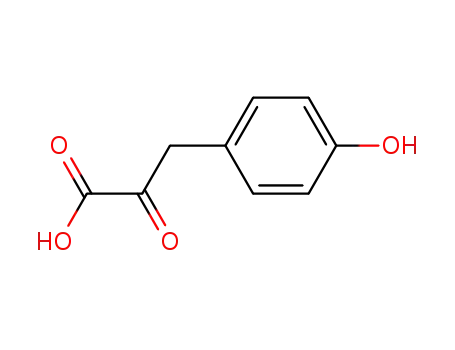
4-hydroxyphenylpiruvic acid

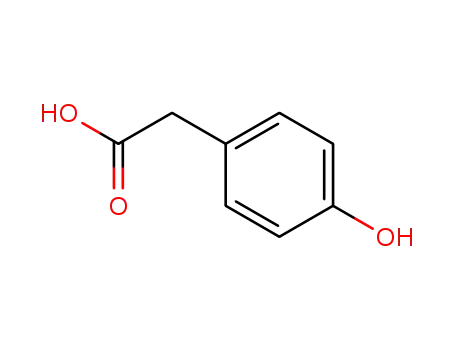
4-hydroxyphenylacetate

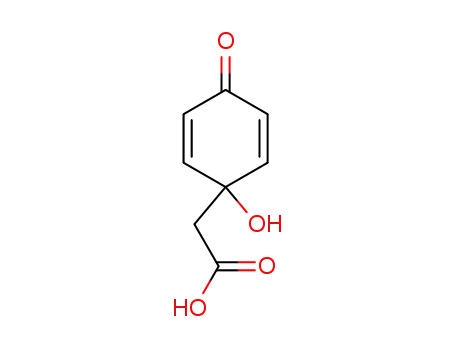
(4-oxo-2,5-cyclohexadien-1-yl)acetic acid

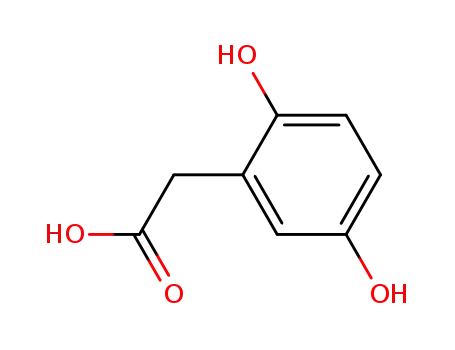
homogentisic acid

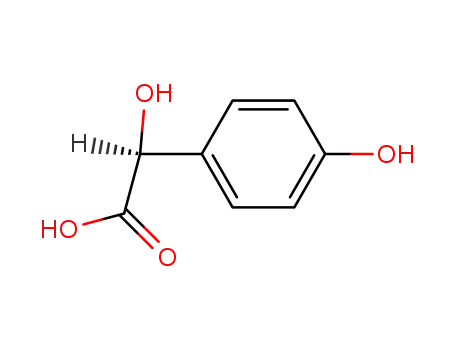
(S)-α,4-dihydroxy-benzeneacetic acid
| Conditions | Yield |
|---|---|
|
With
oxygen; iron(II) sulfate;
In
aq. phosphate buffer;
at 25 ℃;
pH=7;
Enzymatic reaction;
|
47 %Chromat. 19 %Chromat. 15 %Chromat. 20 %Chromat. |

4-hydroxyphenylpiruvic acid


4-hydroxyphenylacetate


(4-oxo-2,5-cyclohexadien-1-yl)acetic acid


homogentisic acid
| Conditions | Yield |
|---|---|
|
With
oxygen; iron(II) sulfate;
In
aq. phosphate buffer;
at 25 ℃;
pH=7;
Reagent/catalyst;
Kinetics;
Enzymatic reaction;
|
8.4 %Chromat. 45 %Chromat. 46.5 %Chromat. |
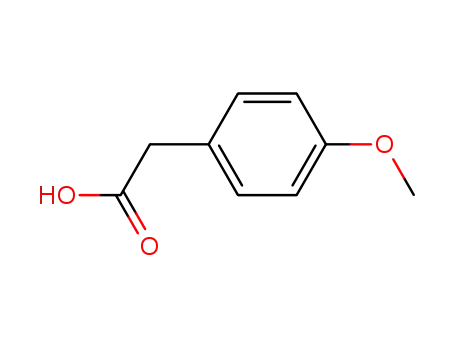
4-Methoxyphenylacetic acid
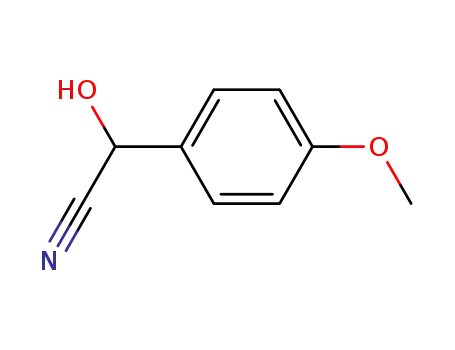
4-methoxymandelonitrile
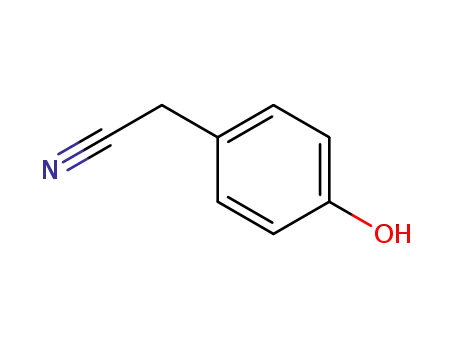
4-cyanomethylphenol
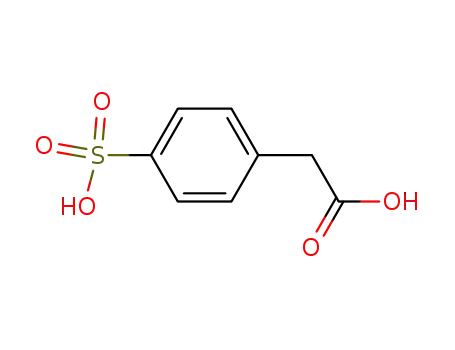
(4-sulfo-phenyl)-acetic acid
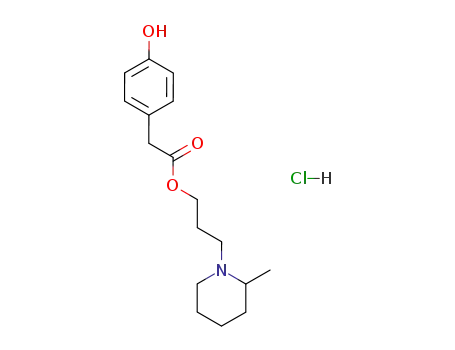
(4-hydroxy-phenyl)-acetic acid-[3-(2-methyl-piperidino)-propylester]; hydrochloride
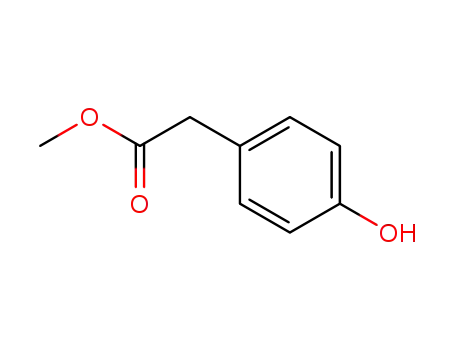
Methyl 4-hydroxyphenylacetate
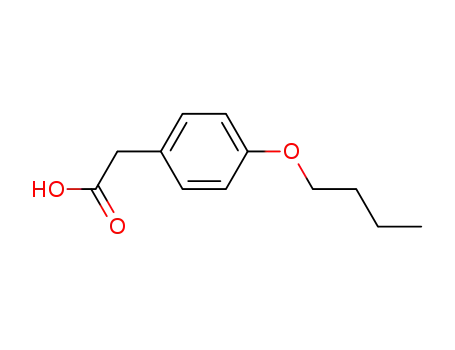
4-butoxyphenylacetic acid
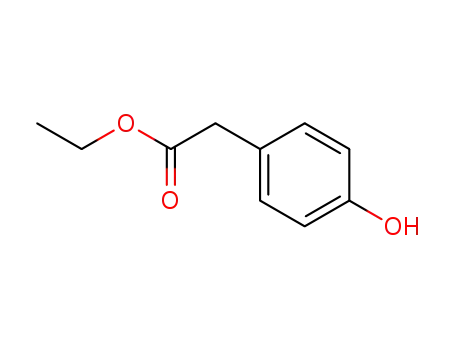
(4-hydroxy-phenyl)-acetic acid ethyl ester