
Antioxidant BHT 264
CAS:128-37-0
Purity:99%
Contact Now
We will contact you as soon as possible
Your Location:Home >Products >Intermediates >553-86-6
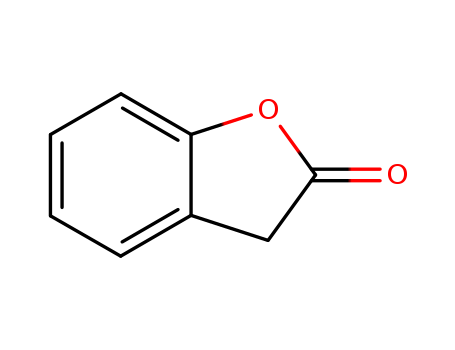

Product Details
|
Reference |
Y. S. Priya, K. Ramachandra Rao, P. V. Chalapathi, M. Satyavani, A. Veeraiah, Vibrational and UV spectroscopic studies of 2-coumaranone by experimental and density functional theory calculations, Journal of Molecular Structure, 2017, vol. 1144, pp. 535-544 |
|
Synthesis Reference(s) |
The Journal of Organic Chemistry, 47, p. 2491, 1982 DOI: 10.1021/jo00133a055 |
InChI:InChI=1/C8H6O2/c9-8-5-6-3-1-2-4-7(6)10-8/h1-4H,5H2
-
The intramolecular cyclisation of 2-hydr...
-
A palladium-catalyzed carbonylative intr...
Pd(II)-catalyzed ortho-C-H acetoxylation...
Reaction of 3-hydroxy-2-pyrones with nit...
Two series of linear extended benzofuran...
The invention discloses a method for imp...
The invention provides a synthetic metho...
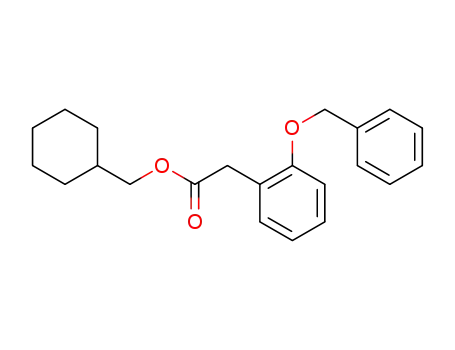
(2-benzyloxyphenyl)acetic acid cyclohexylmethyl ester

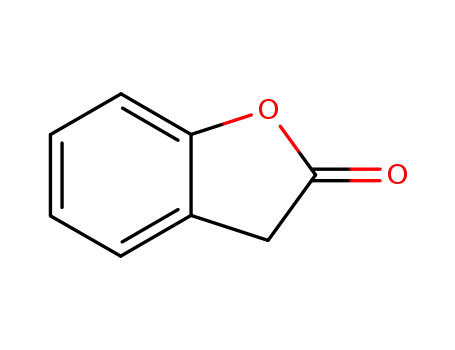
benzofuran-2(3H)-one

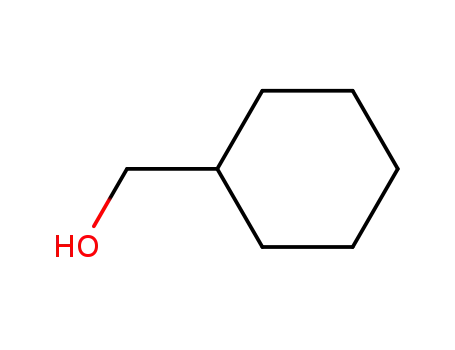
cyclohexylmethyl alcohol
| Conditions | Yield |
|---|---|
|
Multi-step reaction with 2 steps
1: palladium on activated charcoal; hydrogen / ethanol / 20 °C / Inert atmosphere
2: N,N,N',N'-tetramethyl-1,8-diaminonaphthalene / 80 °C / Inert atmosphere
With
palladium on activated charcoal; hydrogen; N,N,N',N'-tetramethyl-1,8-diaminonaphthalene;
In
ethanol;
|
C15H20O3


benzofuran-2(3H)-one


cyclohexylmethyl alcohol
| Conditions | Yield |
|---|---|
|
With
N,N,N',N'-tetramethyl-1,8-diaminonaphthalene;
at 80 ℃;
Inert atmosphere;
|
> 99 %Chromat. |
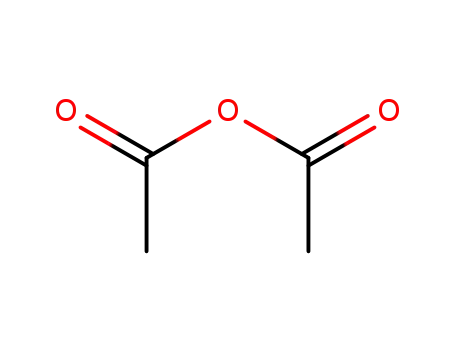
acetic anhydride
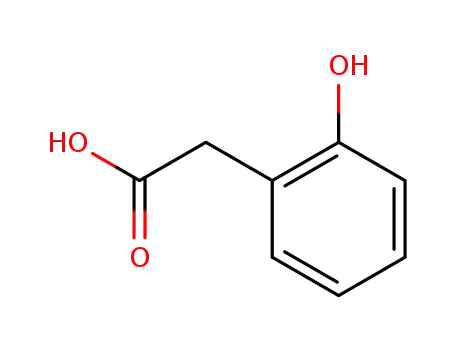
2-Hydroxyphenylacetic acid
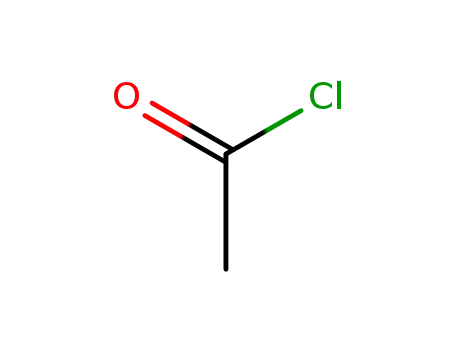
acetyl chloride
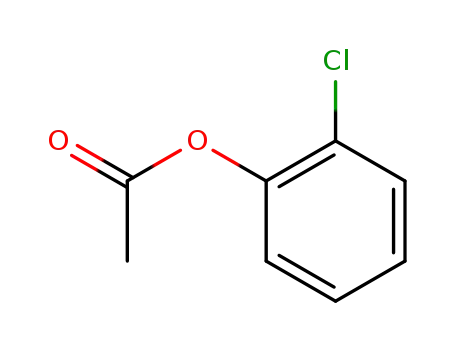
2-chlorophenyl acetate
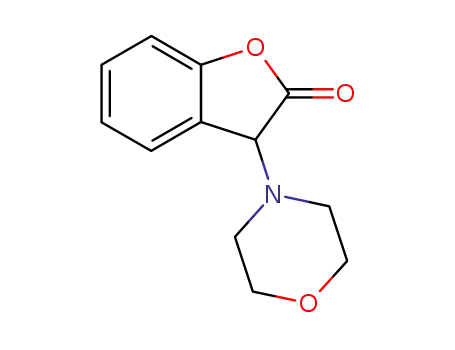
3-morpholino-3H-benzofuran-2-one
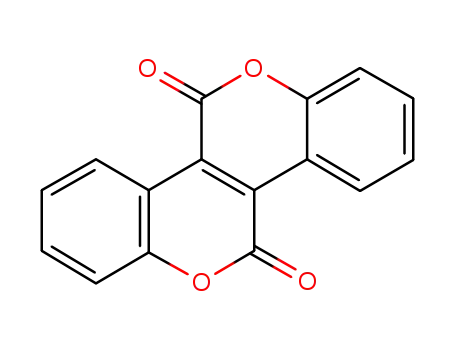
[1]benzopyrano[4,3-c][1]benzopyran-5,11-dione
2-(methoxymethyl)phenol
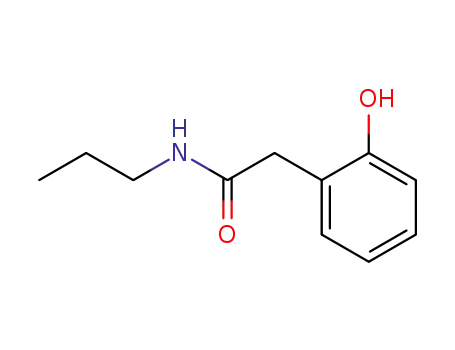
N-Propyl-2-hydroxyphenylacetamid