
Antioxidant BHT 264
CAS:128-37-0
Purity:99%
Contact Now
We will contact you as soon as possible
Your Location:Home >Products >Intermediates >105-67-9

Product Details
|
Use Description |
2,4-Xylenol, a specific chemical compound, serves distinct roles in various fields. In the realm of industrial chemistry, it plays a significant role as a precursor in the synthesis of antioxidants, resins, and specialty chemicals used in various applications, contributing to the enhancement of product stability and performance. In the field of disinfection and sanitation, 2,4-xylenol is utilized as an antimicrobial agent in cleaning products and disinfectants, aiding in the elimination of bacteria and pathogens from surfaces. Moreover, in the fragrance and flavor industries, this compound can serve as a raw material for the creation of scents and flavors that find application in a wide range of products. Its applications in industrial chemistry, disinfection, and fragrances underscore its importance in improving product properties, ensuring hygiene, and enhancing sensory experiences within these distinct domains. |
InChI:InChI=1/C8H10O/c1-6-4-3-5-8(9)7(6)2/h3-5,9H,1-2H3
Sodium perborate in boron trifluoride et...
An iron-NHC complex bearing a tetradenta...
The photochemical reactions of oxygen at...
In this study, a new approach for one-po...
Vapor phaseortho-methylation of 4-chloro...
The specific acid-catalyzed hydrolysis o...
-
A mild and efficient protocol for the sy...
Slow pyrolysis of Eucalyptus grandis woo...
The photolysis of peracetic acid (1) in ...
-
The OsVI nitrido complex, OsVI(N)(quin)2...
-
A series of supported Co modified Mo cat...
Transformation of anisole (methoxybenzen...
Cyclohexa-2,4-dienones stabilized by coo...
A variety of alcohols and phenols, prote...
Benzophenone oxide has been shown to oxi...
-
-
Electrophilic hydroxylation of o- and p-...
-
Enzyme performance can be improved using...
-
In the commercial synthesis of cresols, ...
Ultraviolet irradiation of the title com...
-
In this study, Pb-Cr promoted magnesium ...
A method for the hydroxylation of aryl a...
Catalytic activation of unstrained and n...
The present invention relates to a Fries...
Ceria-promoted Co@NC (NC, N doped carbon...
![sodium[tetrakis(2,4-dimethylphenyloxy)borate]](/upload/2025/1/d5d36b27-7700-4db3-83f9-005175e020fe.png)
sodium[tetrakis(2,4-dimethylphenyloxy)borate]

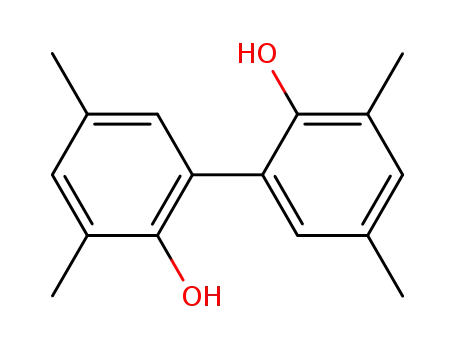
2,2'-dihydroxy-3,3',5,5'-tetramethylbiphenyl

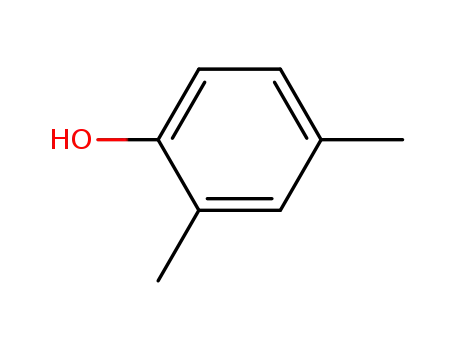
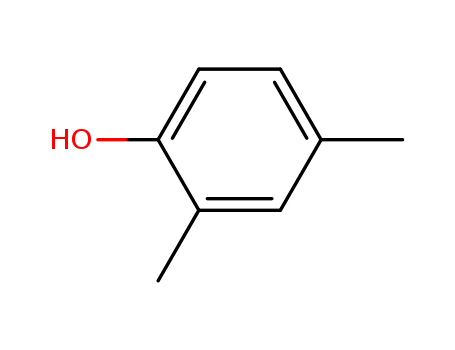
2,4-Xylenol
| Conditions | Yield |
|---|---|
|
sodium[tetrakis(2,4-dimethylphenyloxy)borate];
In
N,N-dimethyl-formamide;
at 20 ℃;
Electrolysis;
With
citric acid;
In
water; N,N-dimethyl-formamide;
for 0.0833333h;
|
65% 2% |
|
sodium[tetrakis(2,4-dimethylphenyloxy)borate];
In
acetonitrile;
at 40 ℃;
Electrolysis;
With
citric acid;
In
water; acetonitrile;
for 0.0833333h;
|
63 % Chromat. 28 % Chromat. |
![sodium[tetrakis(2,4-dimethylphenyloxy)borate]](/upload/2025/1/d5d36b27-7700-4db3-83f9-005175e020fe.png)
sodium[tetrakis(2,4-dimethylphenyloxy)borate]

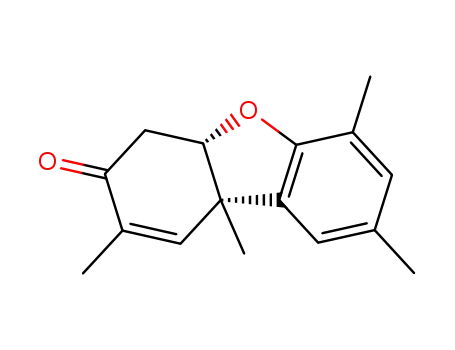
Pummerer's ketone


2,2'-dihydroxy-3,3',5,5'-tetramethylbiphenyl


2,4-Xylenol
| Conditions | Yield |
|---|---|
|
sodium[tetrakis(2,4-dimethylphenyloxy)borate];
In
acetonitrile;
at 20 ℃;
Electrolysis;
With
citric acid;
In
water; acetonitrile;
for 0.0833333h;
|
24% 4% 2% |
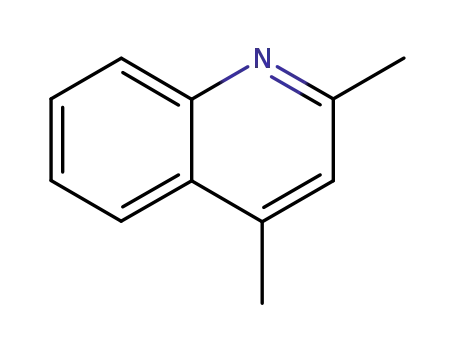
2,4-dimethylquinoline
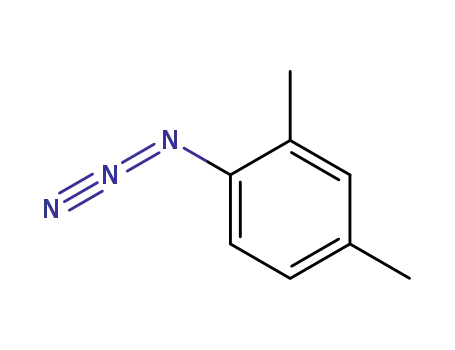
2,4-dimethylphenyl azide
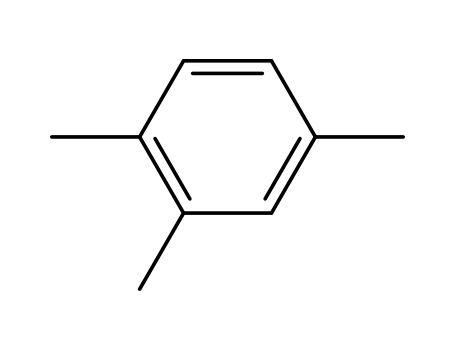
1,2,4-Trimethylbenzene
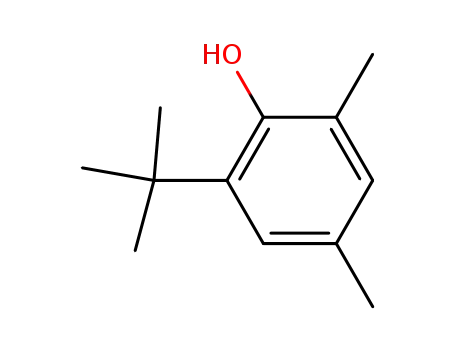
2,4-dimethyl-6-tert-butylphenol
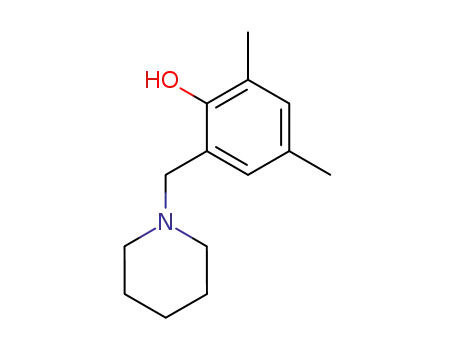
2,4-dimethyl-6-(piperidine-1-yl-methyl)phenol
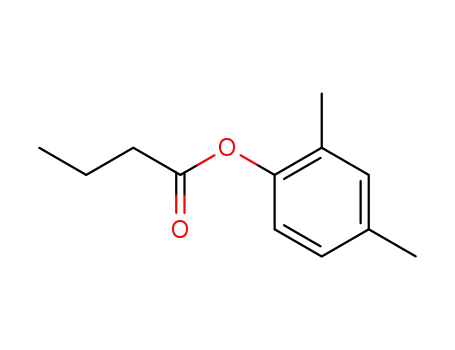
butyric acid-(2,4-dimethyl-phenyl ester)
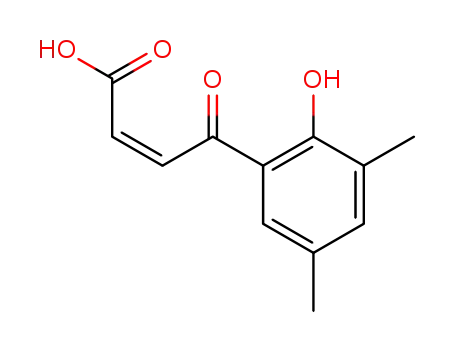
4-(2-hydroxy-3,5-dimethyl-phenyl)-4-oxo-cis-crotonic acid
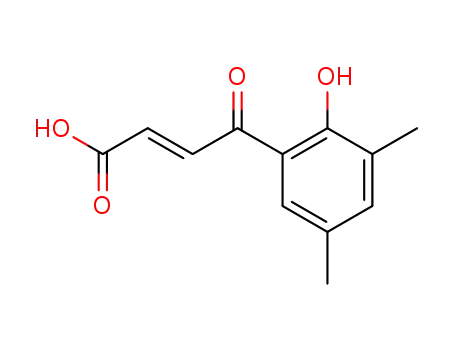
4-(2-hydroxy-3,5-dimethyl-phenyl)-4-oxo-trans-crotonic acid