
Antioxidant BHT 264
CAS:128-37-0
Purity:99%
Contact Now
We will contact you as soon as possible
Your Location:Home >Products >Intermediates >1564-64-3
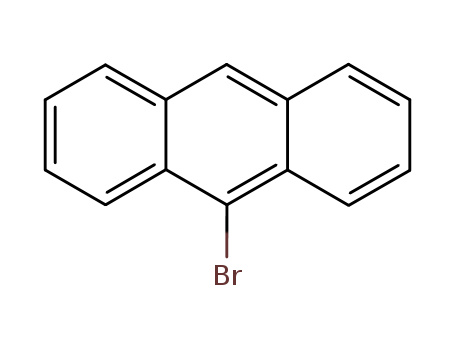

Product Details
|
Preparation |
Anthracene (5 g, 28.05 mmol) was dissolved in CHCl3. Then N-bromosuccinimide (NBS,4.99 g, 28.05 mmol) was added in batches away from light, and the reaction solution was continuously stirred for 12 h. The resulting mixture was stirred for another 30 min with appropriate water, and extracted with CH2Cl2. The CH2Cl2 solution was dried over anhydrous MgSO4. After removing CH2Cl2 solvent, the residue was recrystallized from anhydrous ethanol to give 4.78 g (66.3 %) of a green-yellow needle solid. 1H NMR (500 MHz, CDCl3) δ 8.55 (d, J = 8.9 Hz, 2H), 8.48 (s, 1H), 8.03 (d, J = 8.4 Hz, 2H), 7.67 – 7.60 (m, 2H), 7.56 – 7.51 (m, 2H). EI-MS (m/z): Calculated for C14H9Br: 257.13. Found [M+ ]: 255.96. Synthesis of 9-bromoanthracene |
|
Synthesis Reference(s) |
The Journal of Organic Chemistry, 57, p. 2740, 1992 DOI: 10.1021/jo00035a038 |
|
Purification Methods |
Crystallise 9-bromoanthracene from MeOH or EtOH followed by sublimation in vacuo. [Masnori et al. J Am Chem Soc 108 126 1986, Beilstein 5 IV 2295.] |
InChI:InChI=1/C14H9Br/c15-14-12-7-3-1-5-10(12)9-11-6-2-4-8-13(11)14/h1-9H
Picosecond laser photolysis reveals the ...
The photochemical reactions of 1,2,3,4-t...
A one-step postpolymerization modificati...
-
-
Lewis acids are frequently employed in c...
Bromoisobutyrate has been used for the f...
Alkoxyamide has been reported as a catal...
A mild, metal-free bromination method of...
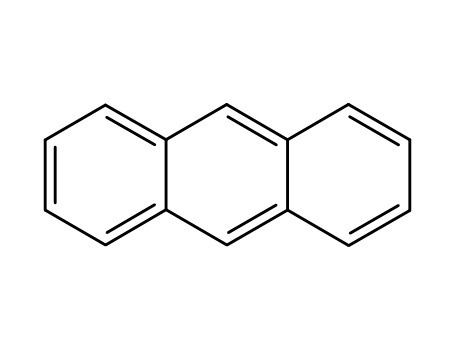
anthracene

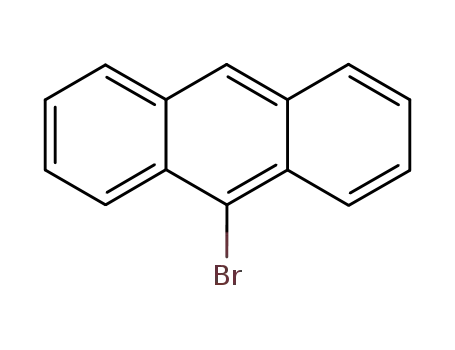
9-Bromoanthracene

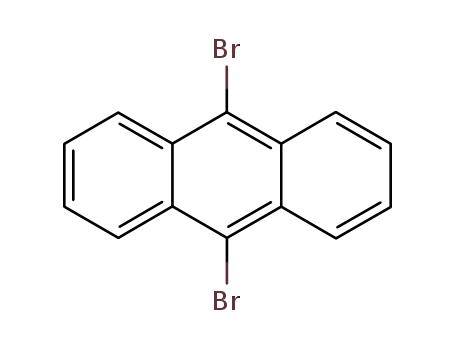
9,10-Dibromoanthracene
| Conditions | Yield |
|---|---|
|
With
N-Bromosuccinimide; 2,4,6-trimethylaniline;
In
dichloromethane;
at 0 ℃;
for 8h;
regioselective reaction;
Inert atmosphere;
|
84% 7% |
|
With
N-Bromosuccinimide; C5H13NO3S;
In
n-heptane;
at 60 ℃;
for 4.5h;
Darkness;
|
81% 8% |
|
With
N-Bromosuccinimide; iodine;
In
tetrachloromethane;
Heating;
|
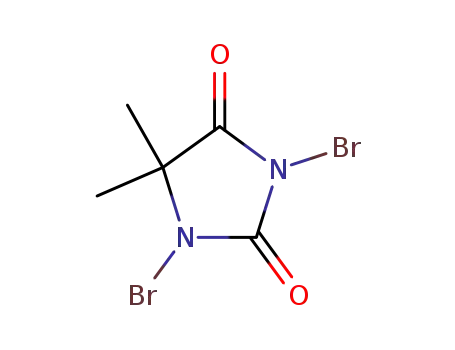
1,3-dibromo-5,5-dimethylimidazolidine-2,4-dione


anthracene


9-Bromoanthracene


9,10-Dibromoanthracene
| Conditions | Yield |
|---|---|
|
1,3-dibromo-5,5-dimethylimidazolidine-2,4-dione; anthracene;
In
ethyl acetate;
for 2.5h;
Refluxing;
In
tetrahydrofuran;
|
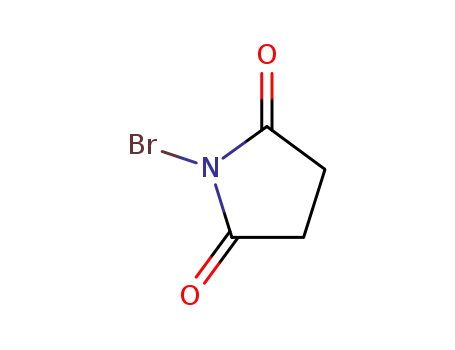
N-Bromosuccinimide

anthracene
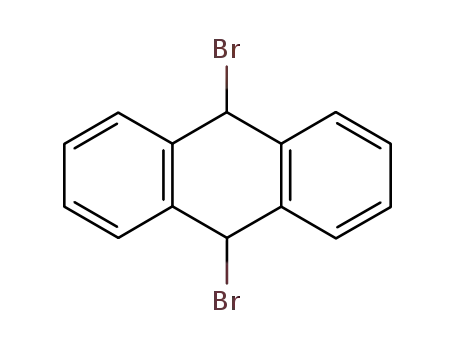
9,19-Dibromo-9,10-dihydroanthracene
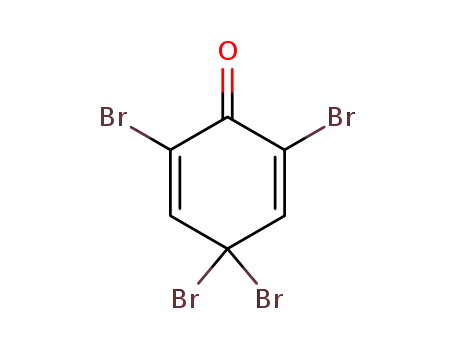
2,4,4,6-Tetrabromo-2,5-cyclohexadien-1-one
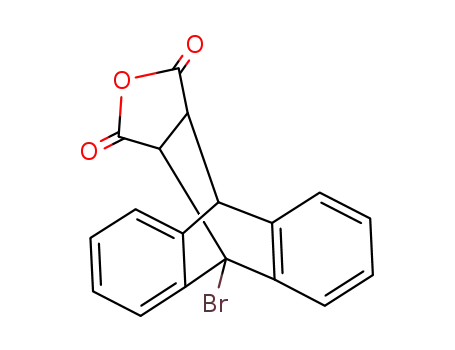
9-bromo-9,10-dihydro-9,10-ethano-anthracene-11,12-dicarboxylic acid-anhydride
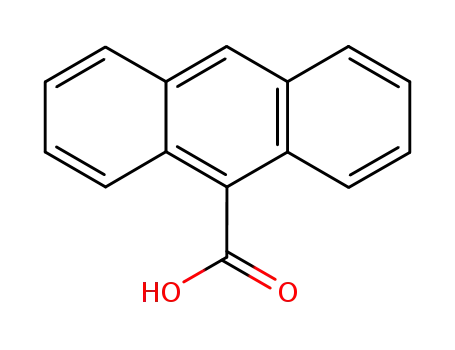
anthracen-9-carboxylic acid
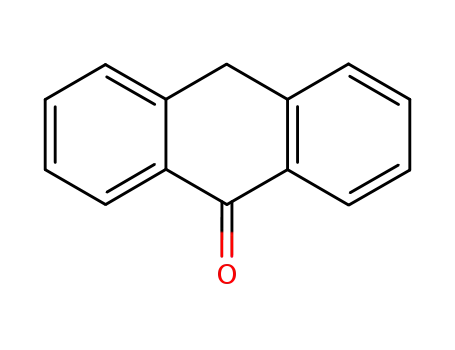
anthracen-9(10H)-one

9,10-Dibromoanthracene