
Antioxidant BHT 264
CAS:128-37-0
Purity:99%
Contact Now
We will contact you as soon as possible
Your Location:Home >Products >Intermediates >573-17-1
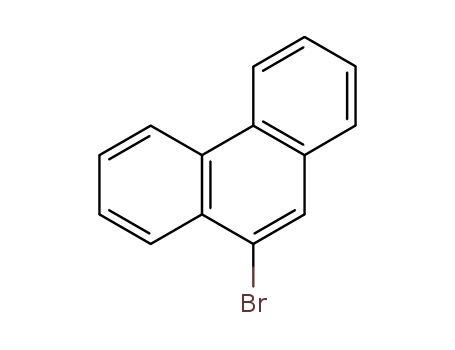

Product Details
|
Preparation |
9-Bromophenanthrene (90-94%) is produced by adding bromine to a refluxing solution of phenanthrene in carbon tetrachloride. This is the starting-point of 9-substituted phenanthrenes, e.g, when heated with cuprous cyanide at 260℃, 9-bromophenanthrene forms the corresponding cyano-compound; this may be hydrolysed to phenanthrene-9-carboxylic acid. Phenanthrene undergoes the Friedel-Crafts reaction mainly in the 3-, and to a small extent, in the 2-position. It is chloromethylated in the 9-position. When nitrated, phenanthrene gives a mixture of three mononitro-derivatives, the 3-isomer predominating. Sulphonation of phenanthrene gives a mixture of 1-, 2-, 3- and 9-phenanthrenesulphonic acids, and the ratio of these isomers depends on the temperature. |
InChI:InChI=1/C14H9Br/c15-14-9-10-5-1-2-6-11(10)12-7-3-4-8-13(12)14/h1-9H
ortho-Silylaryl triflate precursors (oSA...
The invention discloses a preparation me...
A transition metal-free synthesis of ena...
Combined use of oxorhenium catalysts wit...
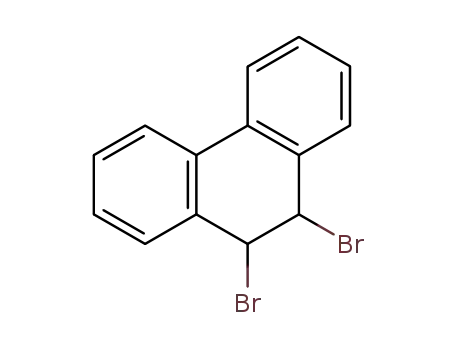
9,10-dibromo-9,10-dihydro-phenanthrene

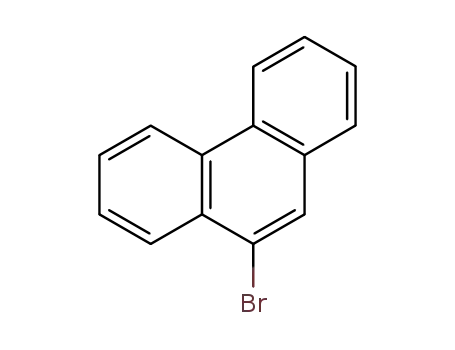
9-bromophenanthrene

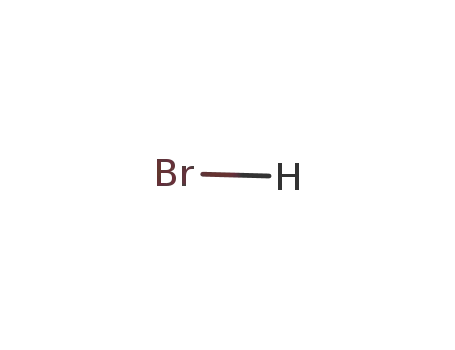
hydrogen bromide
| Conditions | Yield |
|---|---|
|
beim Erhitzen fuer sich;
|

9,10-dibromo-9,10-dihydro-phenanthrene

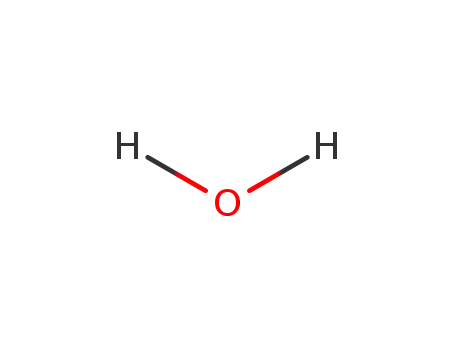
water


9-bromophenanthrene

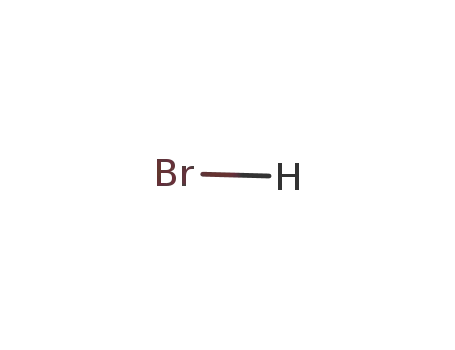
hydrogen bromide
| Conditions | Yield |
|---|---|
|
im geschlossenen Rohr;
|
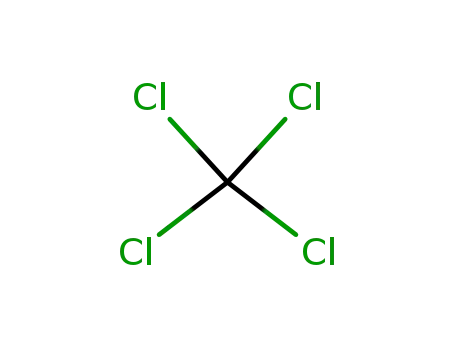
tetrachloromethane
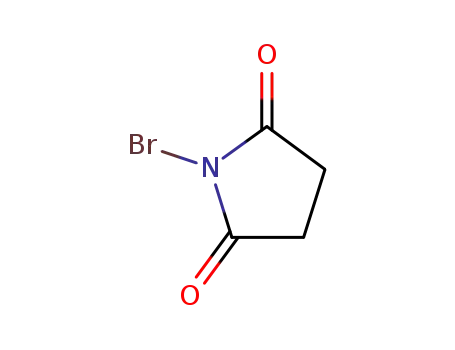
N-Bromosuccinimide
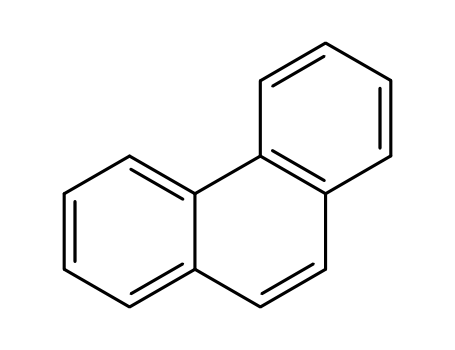
phenanthrene

9,10-dibromo-9,10-dihydro-phenanthrene

phenanthrene
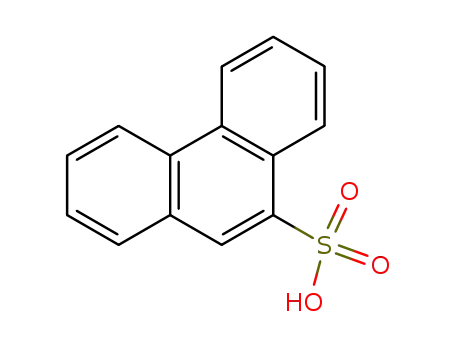
phenanthrene-9-sulfonic acid
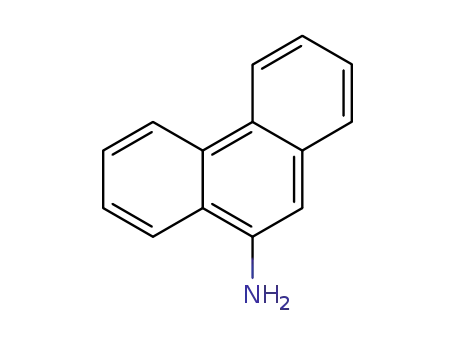
9-Aminophenanthrene
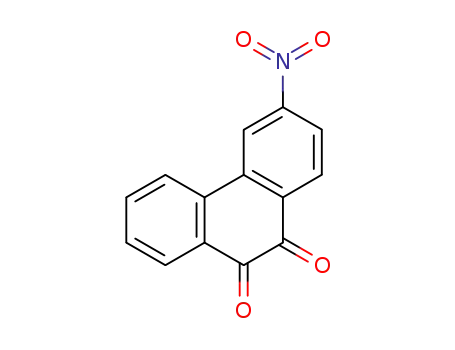
3-Nitrophenanthraquinone