
Antioxidant BHT 264
CAS:128-37-0
Purity:99%
Contact Now
We will contact you as soon as possible
Your Location:Home >Products >Intermediates >215527-70-1

Product Details
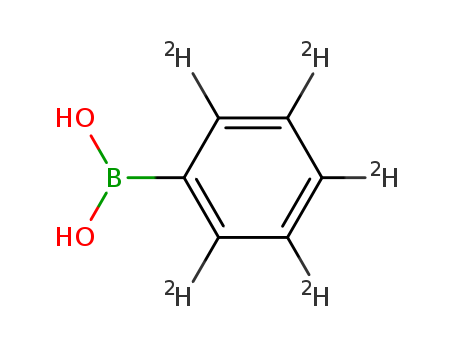
InChI:InChI=1/C6H7BO2/c8-7(9)6-4-2-1-3-5-6/h1-5,8-9H/i1D,2D,3D,4D,5D
A new type of domino reaction for synthe...
Two higly stereospecific microsomal cyto...
Herein, electrochemically driven, Rh(iii...
An unprecedented Pd(II)-catalyzed decarb...
The invention relates to the field of or...
An arylation/intramolecular conjugate ad...
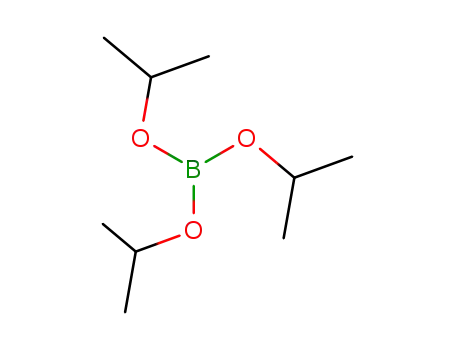
Triisopropyl borate

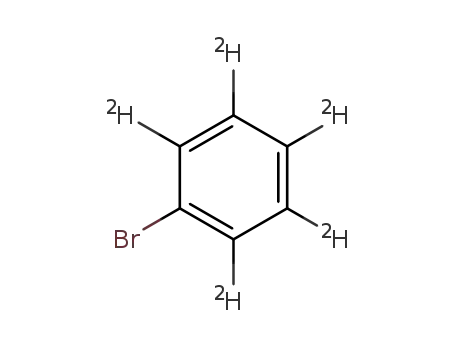
bromobenzene-d5

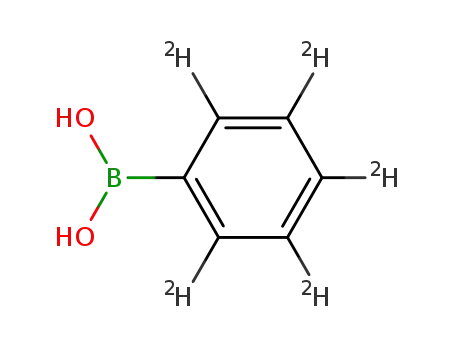
2,3,4,5,6-pentadeuteriumbenzeneboronic acid
| Conditions | Yield |
|---|---|
|
bromobenzene-d5;
With
n-butyllithium;
In
tetrahydrofuran; hexane;
at -78 ℃;
for 2h;
Inert atmosphere;
Triisopropyl borate;
In
tetrahydrofuran; hexane;
at -78 - 20 ℃;
|
84% |
|
bromobenzene-d5;
With
n-butyllithium;
In
tetrahydrofuran; hexane;
at -78 ℃;
for 2h;
Inert atmosphere;
Triisopropyl borate;
In
tetrahydrofuran; hexane;
at 20 ℃;
for 3h;
|
64% |
|
bromobenzene-d5;
With
n-butyllithium;
In
tetrahydrofuran;
at -78 ℃;
for 2.5h;
Inert atmosphere;
Triisopropyl borate;
In
tetrahydrofuran;
at -78 - 20 ℃;
Inert atmosphere;
With
hydrogenchloride; water;
In
tetrahydrofuran;
at 20 ℃;
for 3h;
|
|
|
bromobenzene-d5;
With
n-butyllithium;
In
tetrahydrofuran;
at -78 ℃;
for 2.5h;
Inert atmosphere;
Schlenk technique;
Triisopropyl borate;
In
tetrahydrofuran;
at -78 - 20 ℃;
Inert atmosphere;
Schlenk technique;
With
hydrogenchloride;
In
tetrahydrofuran; water;
at 20 ℃;
for 3h;
Inert atmosphere;
Schlenk technique;
|
|
|
bromobenzene-d5;
With
n-butyllithium;
In
tetrahydrofuran; hexane;
at -78 ℃;
for 0.25h;
Inert atmosphere;
Triisopropyl borate;
In
tetrahydrofuran; hexane;
at 25 ℃;
for 0.333333h;
Inert atmosphere;
|
|
|
bromobenzene-d5;
With
n-butyllithium;
In
tetrahydrofuran;
at -78 ℃;
for 1.25h;
Inert atmosphere;
Triisopropyl borate;
In
tetrahydrofuran;
at 20 ℃;
for 12h;
Inert atmosphere;
|
|
|
bromobenzene-d5;
With
n-butyllithium;
In
tetrahydrofuran;
at -78 ℃;
for 0.5h;
Inert atmosphere;
Triisopropyl borate;
In
tetrahydrofuran;
at -78 ℃;
for 2h;
Inert atmosphere;
|
|
|
bromobenzene-d5;
With
n-butyllithium;
In
tetrahydrofuran;
at -78 ℃;
for 2.5h;
Inert atmosphere;
Triisopropyl borate;
In
tetrahydrofuran;
at -78 - 20 ℃;
Inert atmosphere;
|
|
|
bromobenzene-d5;
With
n-butyllithium;
In
tetrahydrofuran;
at -78 ℃;
for 2h;
Inert atmosphere;
Triisopropyl borate;
In
tetrahydrofuran;
at -78 - 20 ℃;
Inert atmosphere;
|
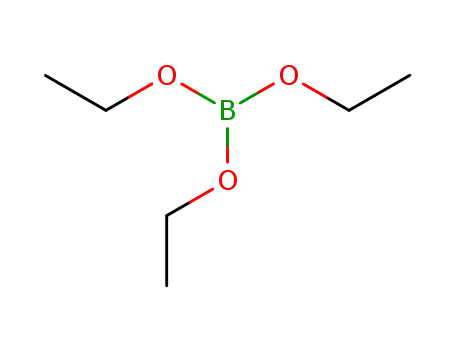
triethyl borate


bromobenzene-d5


2,3,4,5,6-pentadeuteriumbenzeneboronic acid
| Conditions | Yield |
|---|---|
|
bromobenzene-d5;
With
tert.-butyl lithium;
In
tetrahydrofuran;
at -78 ℃;
for 0.5h;
triethyl borate;
In
tetrahydrofuran;
at -78 - 20 ℃;
for 12h;
With
hydrogenchloride;
In
tetrahydrofuran; water;
for 1h;
|
69% |
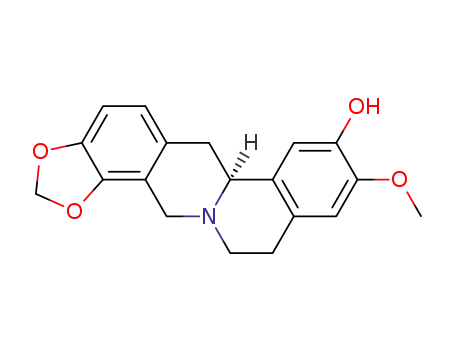
(6aS)-6,11,12,14-tetrahydro-9-methoxy-6aH-[1,3]dioxolo[4,5-h]isoquinolino[2,1-b]isoquinolin-8-ol

bromobenzene-d5

triethyl borate
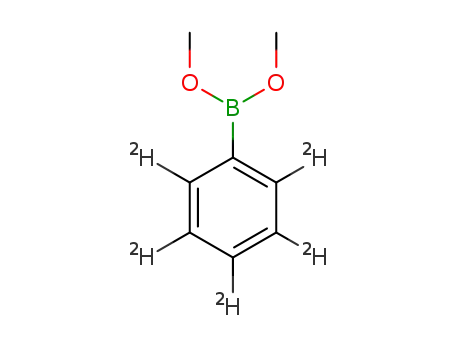
C8H6(2)H5BO2
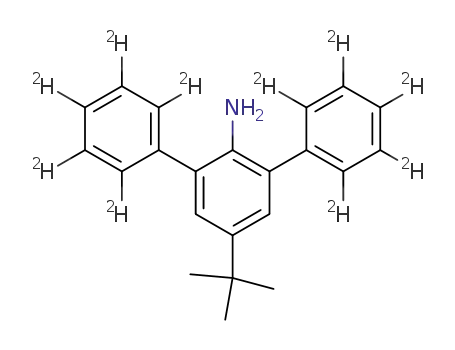
4-tert-butyl-2,6-di(phenyl-d5)aniline
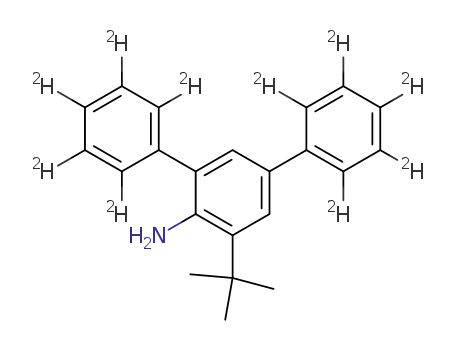
(2,4-Diphenyl-d5)-6-tert-butylaniline
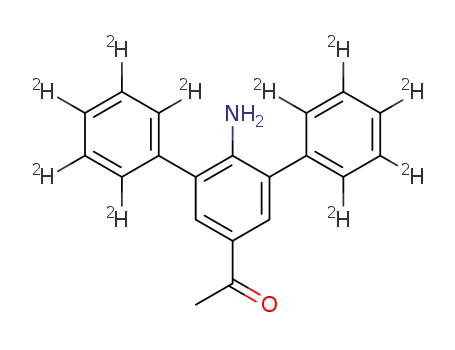
4-acetyl-2,6-di(phenyl-d5)aniline
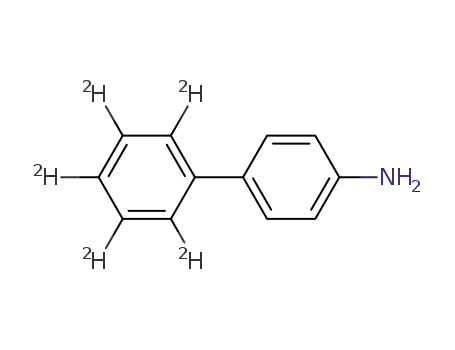
4-(phenyl-d5)aniline