
Antioxidant BHT 264
CAS:128-37-0
Purity:99%
Contact Now
We will contact you as soon as possible
Your Location:Home >Products >High-Tech New Material >22560-16-3

Product Details
|
Preparation |
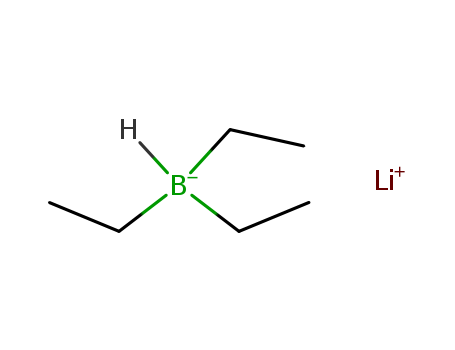
LiBHEt3 is prepared by the reaction of lithium hydride (LiH) and triethylborane (Et3B) in tetrahydrofuran (THF):LiH + Et3B → LiEt3BHIts THF solutions are stable indefinitely in the absence of moisture and air. |
|
Precautions |
Moisture sensitive. Air sensitive. Incompatible with water and strong oxidizing agents. |
|
Application |
Lithium Triethylborohydride (LiTEBH) is widely used as a powerful and selective reducing agent that shows super hydride activity in organic synthesis.LiTEBH can be used as a reagent:To reduce alkyl halides to alkanes via dehydrogenation reactions.For the selective reduction of epoxides to Markovnikov alcohols.To reduce tosylates or mesylates primary alcohols to hydrocarbons.For reductive cyclization reactions for the preparation of useful intermediates.In the synthesis of hepta(manno-3-deoxy-6-O-t-butyldimethylsilyl)-β-cyclodextrin by reduction of hepta(manno-2,3-anhydro-6-O-t-butyldimethylsilyl)-β-cyclodextrin.For hydrodefluorination of C-F bonds using Ni catalyst.To prepare alkynyl alcohols from cleavage of cyclic keto-vinyl triflates.In the stereoselective reduction of bicyclic imides, isoquinolines, and pyridines.To prepare tungsten and molybdenum hydride complexes. |
|
General Description |
Super-Hydride? solution (Lithium triethylborohydride or LiTEBH) is widely used as a powerful and selective reducing agent that shows super hydride activity in organic synthesis. |
InChI:InChI=1/C6H16B.Li/c1-4-7(5-2)6-3;/h7H,4-6H2,1-3H3;/q-1;+1
The present invention relates to novel o...
The reaction of t-butyllithium with repr...
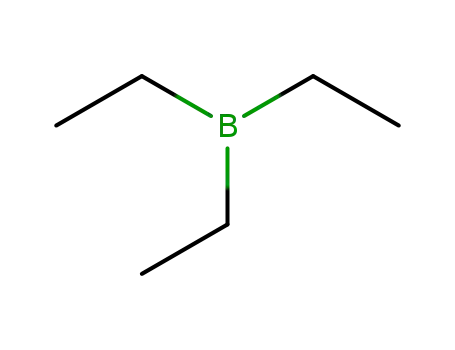
triethyl borane


lithium hydride

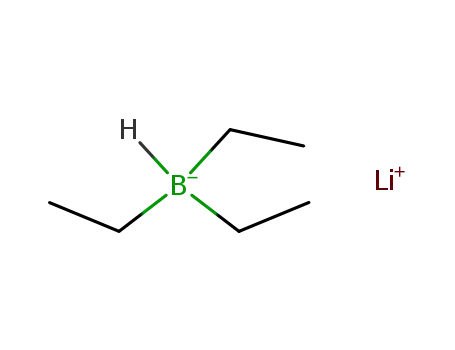
lithium triethylborohydride
| Conditions | Yield |
|---|---|
|
in an autoclave near 200°C;
|
|
|
in an autoclave near 200°C;
|
|
|
In
tetrahydrofuran;
|

triethyl borane


lithium triethylborohydride
| Conditions | Yield |
|---|---|
|
With
n-butyllithium;
In
tetrahydrofuran; pentane;
byproducts: isobutylene; (N2); to stirred soln. of boranederiv. in THF was dropwise added pentane soln. of LiBu at -78°C, then mixt. was allowed to warm up to room temp.; not isolated, detected by (11)B-NMR;
|
>99 |

triethyl borane
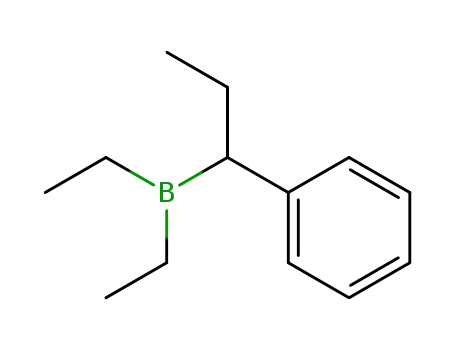
Diethyl-(1-phenylpropyl)borane
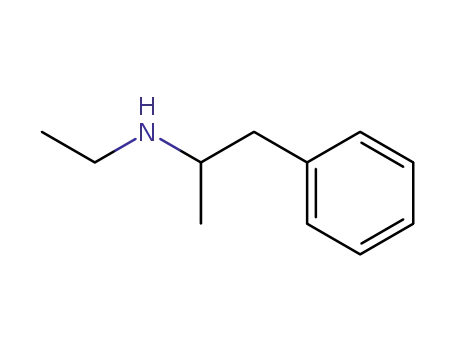
N-ethylamphetamine
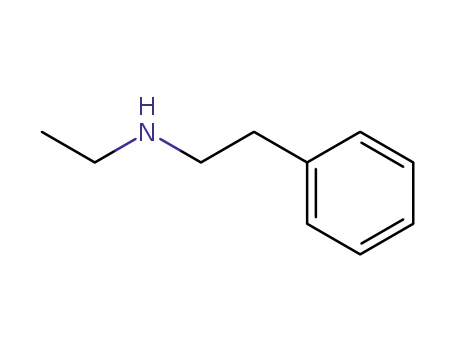
N-ethyl-2-phenylethanamine
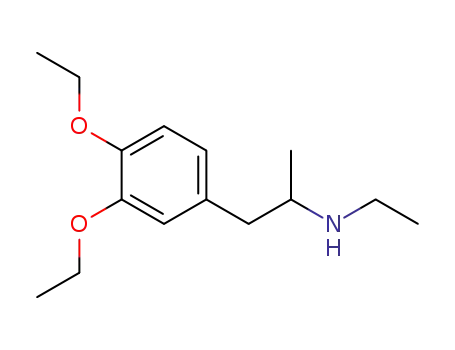
[2-(3,4-Diethoxy-phenyl)-1-methyl-ethyl]-ethyl-amine