
Antioxidant BHT 264
CAS:128-37-0
Purity:99%
Contact Now
We will contact you as soon as possible
Your Location:Home >Products >Pharmaceutical API >2078-54-8

Product Details
|
World Health Organization (WHO) |
Propofol, a short acting injectable anaesthetic, was introduced in 1987. In April 1992, the Norwegian Medicines Control Board reported that prolonged use of propofol had been associated with two fatalities in children characterized by metabolic acidosis, liver enlargement, and cerebral oedema. The UK Committee on the Safety of Medicines has received 5 reports of deaths occurring in children who had received propofol while in intensive care. |
|
Biological Functions |
Propofol (Diprivan) is rapidly acting, has a short recovery time, and possesses antiemetic properties. A rapid onset of anesthesia (50 seconds) is achieved, and if no other drug is administered, recovery will take place in 4 to 8 minutes.The recovery is attributed to redistribution of the drug and rapid metabolism to glucuronide and sulfate conjugates by the liver and extrahepatic tissues, such as intestine and kidney. Rapid recovery and its antiemetic properties make propofol anesthesia very popular as an induction agent for outpatient anesthesia. Propofol can also be used to supplement inhalational anesthesia in longer procedures. Both continuous infusion of propofol for conscious sedation and with opioids for the maintenance of anesthesia for cardiac surgery are acceptable techniques. |
|
Synthesis Reference(s) |
The Journal of Organic Chemistry, 21, p. 712, 1956 DOI: 10.1021/jo01112a621 |
|
Biological Activity |
Intravenous general anesthetic and hypnotic with a mode of action which includes potentiation of GABA-mediated inhibitory synaptic transmission, direct activation of the GABA A receptor and inhibition of glutamate receptor mediated excitatory synaptic transmission. Also potentiates P2X 4 receptor-mediated currents in P2X 4 -HEK293 cells. |
|
Pharmacology |
Propofol is primarily a hypnotic drug with substantial cardiorespiratory depressant actions and with no ability to produce neuromuscular blockade. While propofol lacks analgesic properties, its use permits lower doses of opioids. Likewise, less propofol is required for adequate hypnosis when it is administered with opioids.Thus, it is said that propofol and opioids interact synergistically. |
|
Side effects |
The dose of propofol should be reduced in older patients; however, it does have a relatively linear dose– response characteristic, and patients generally can be safely titrated. The pain on injection, especially when small veins are used, can be considerably reduced if lidocaine 20 mg is administered first. Anesthesia induction with propofol causes a significant reduction in blood pressure that is proportional to the severity of cardiovascular disease or the volume status of the patient, or both. However, even in healthy patients a significant reduction in systolic and mean arterial blood pressure occurs. The reduction in pressure appears to be associated with vasodilation and myocardial depression. Although propofol decreases systemic vascular resistance, reflex tachycardia is not observed. This is in contrast to the actions of thiopental. The heart rate stabilization produced by propofol relative to other agents is likely the result of either resetting or inhibiting the baroreflex, thus reducing the tachycardic response to hypotension. Since propofol does not depress the hemodynamic response to laryngoscopy and intubation, its use may permit wide swings in blood pressure at the time of induction of anesthesia. Propofol should be used with utmost caution in patients with cardiac disease. |
|
Safety Profile |
Poison by intravenous and intraperitoneal routes. Experimental reproductive effects. Combustible when exposed to heat or flame; can react with oxidizing materials. To fight fire, use foam, CO2, dry chemical. When heated to decomposition it emits acrid smoke and fumes. See also PHENOL |
|
Veterinary Drugs and Treatments |
In appropriate patients, propofol may be useful as an induction agent (especially before endotracheal intubation or an inhalant anesthetic), and as an anesthetic for outpatient diagnostic or minor procedures (e.g., laceration repair, radiologic procedures, minor dentistry, minor biopsies, endoscopy, etc.). Propofol is used as a treatment for refractory status epilepticus, as it tends to cause less cardiovascular depression and recoveries can be smoother than with pentobarbital. Propofol may be of particular usefulness for use in Greyhounds and in patients with preexisting cardiac dysrhythmias. At low dosages, propofol is being investigated as an appetite stimulant in dogs. Propofol may be safely used in animals with liver or renal disease and mild to moderate cardiac disease. In dogs, propofol’s labeled indications are: 1) for induction of anesthesia; 2) for maintenance of anesthesia for up to 20 minutes; 3) for induction of general anesthesia where maintenance is provided by inhalant anesthetics. |
|
Brand name |
Diprivan (Abraxis);Disoprivan;Disprofol;Rapinovet. |
|
General Description |
Propofol is an injectable sedative–hypnotic used for the inductionand maintenance of anesthesia or sedation. Propofolis only slightly soluble in water with an octanol/water partitioncoefficient of 6,761:1; thus, it is formulated as an oil-inwateremulsion. The fat component of the emulsion consistsof soybean oil, glycerol, and egg lecithin. The pKa of thepropanol hydroxyl is 11 and the injectable emulsion has apH of 7 to 8.5. Formulations contain either disodium ethylenediaminetetraaceticacid (EDTA) (0.005%) or sodiummetabisulfite to retard the growth of microorganisms. EDTAis a metal chelator and patients on propofol containingEDTA for extended periods of time excrete more zinc andiron in their urine. The clinical consequence of this is notknown but the manufacturer recommends that a drug holidayor zinc supplementation be considered after 5 days of therapy. |
InChI:InChI=1/C12H18O/c1-8(2)10-6-5-7-11(9(3)4)12(10)13/h5-9,13H,1-4H3
Phenol can be alkylated with propan-2-ol...
Propofol (2,6-diisopropylphenol/DIPP) is...
HMCM-49/MCM-41 composite molecular sieve...
Al-MCM-48 molecular sieves (Si/Al molar ...
The invention discloses a synthesis meth...
A general method for the demethylation, ...
Herein, we report a continuous flow proc...
A process for manufacturing Pure Propofo...
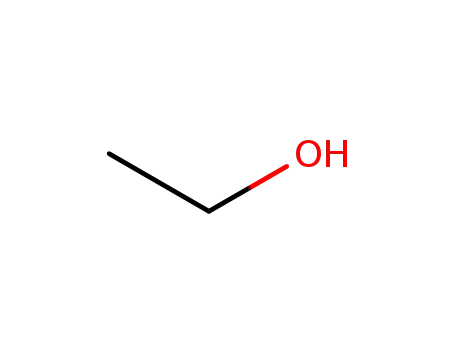
ethanol

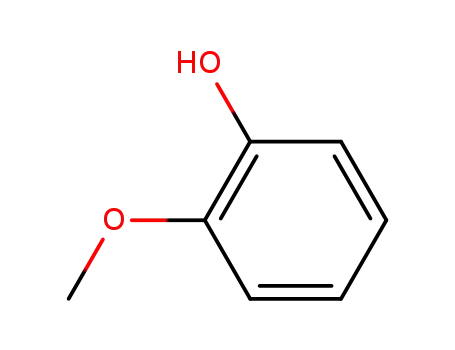
2-methoxy-phenol

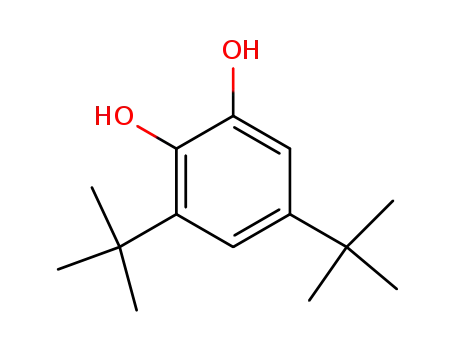
3,5-Di-tert-butylcatechol

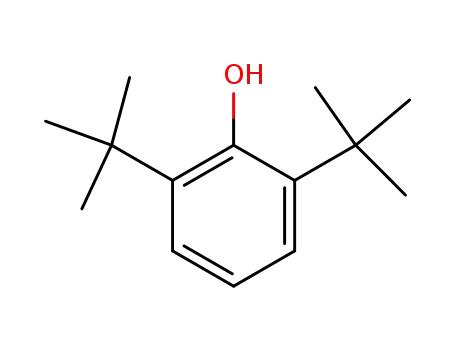
2,6-di-tert-butylphenol

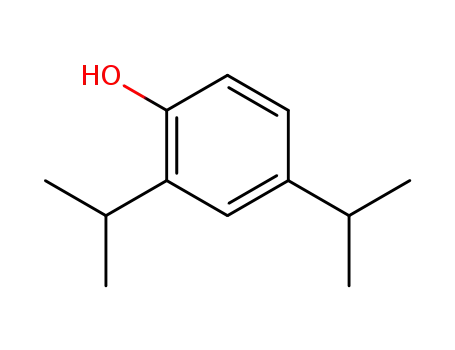
2,4-diisopropylphenol

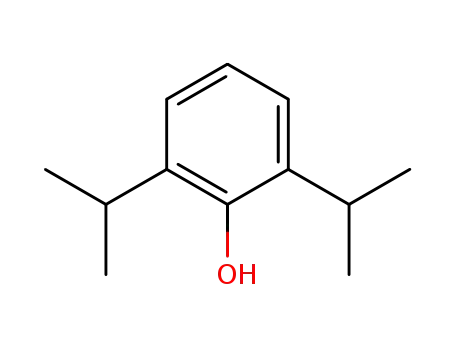
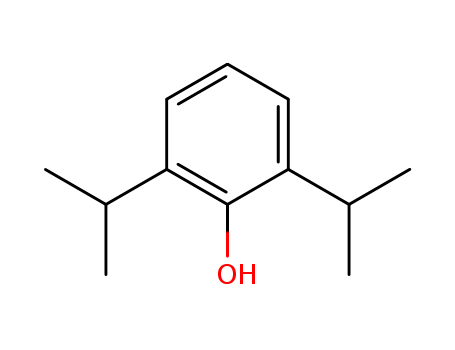
2,6-diisopropylphenol

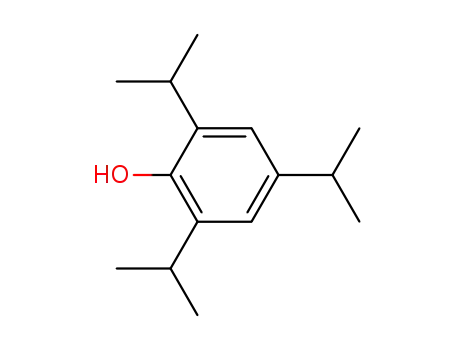
2,4,6-triisopropylphenol

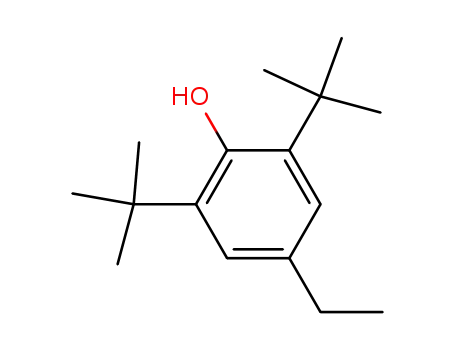
2,6-di-tert-butyl-4-ethylphenol

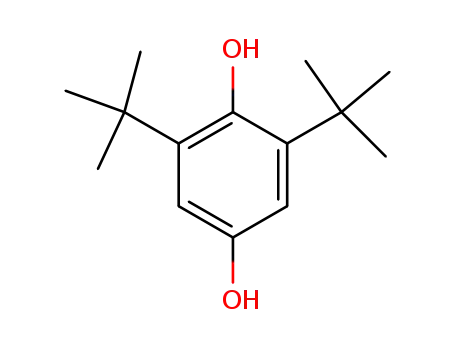
2,6-di-tert-butyl-4-hydroxyphenol

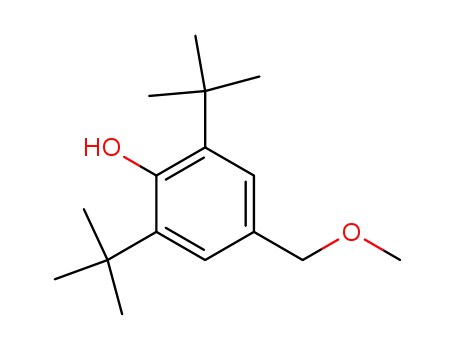
2,6-di-tert-butyl-4-methoxymethylene-phenol

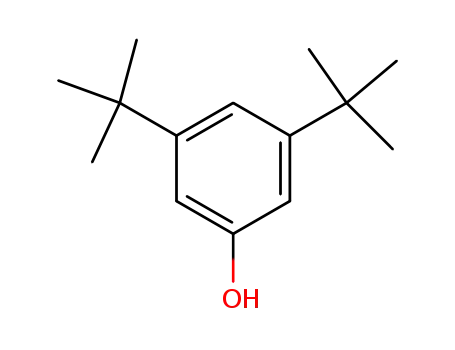
3,5-Di-tert-butylphenol

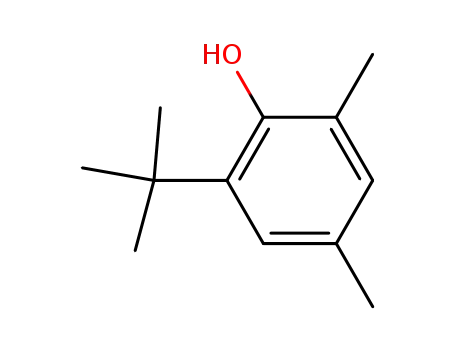
2,4-dimethyl-6-tert-butylphenol

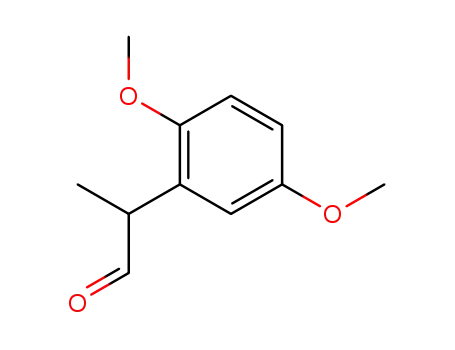
2-(2',5'-dimethoxyphenyl)propionaldehyde

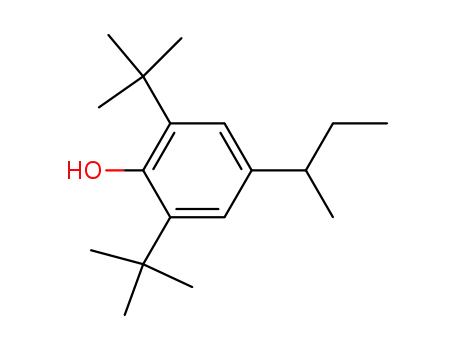
2,6-di-tert-butyl-4-sec-butylphenol

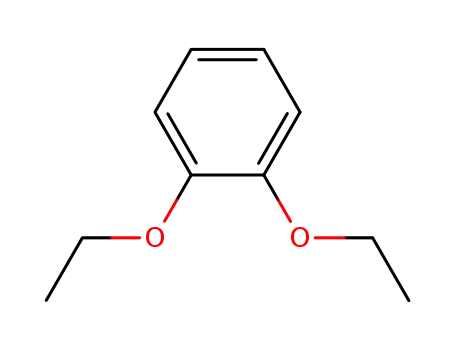
1,2-diethoxybenzene

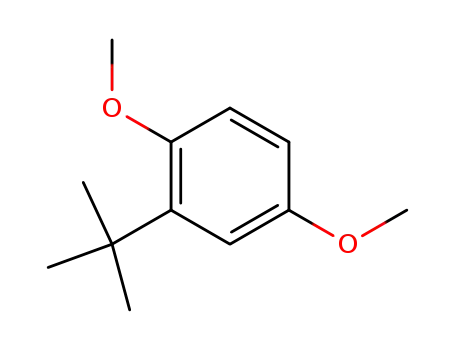
1,4-dimethoxy-2-tert-butylbenzene

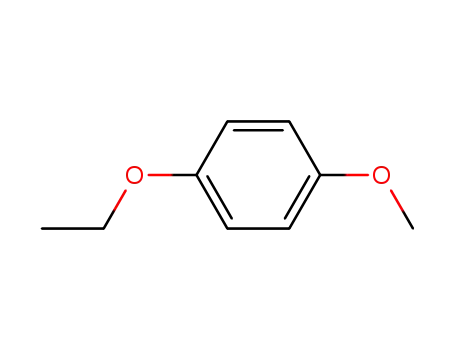
1-ethoxy-4-methoxybenzene

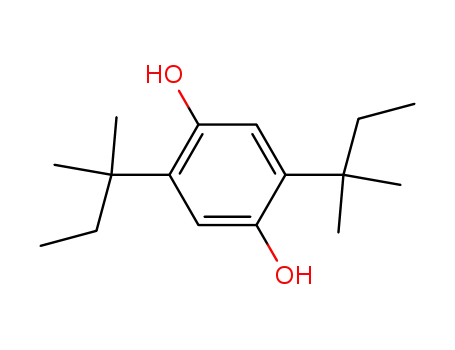
2,5-di(tert-amyl)-1,4-hydroquinone

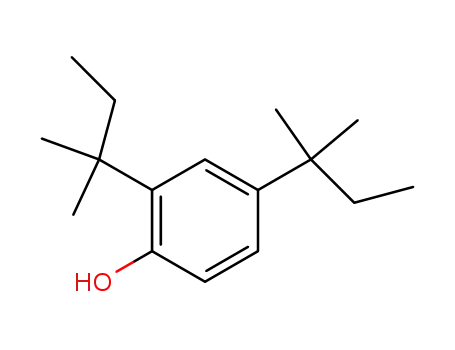
2,4-di-tert-amylphenol

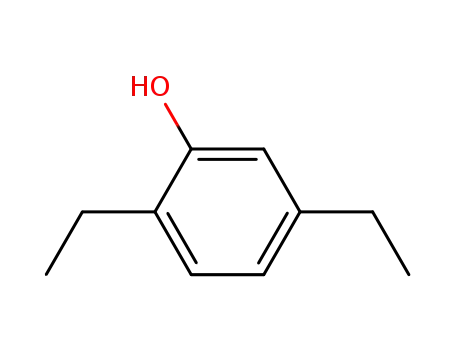
2,5-diethyl phenol

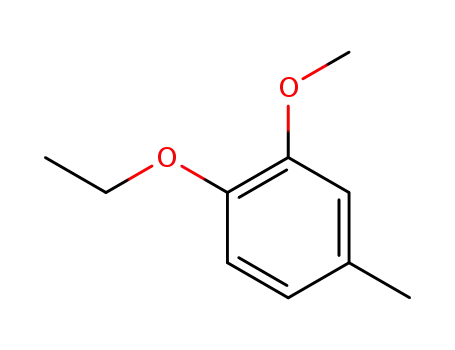
4-ethoxy-3-methoxytoluene

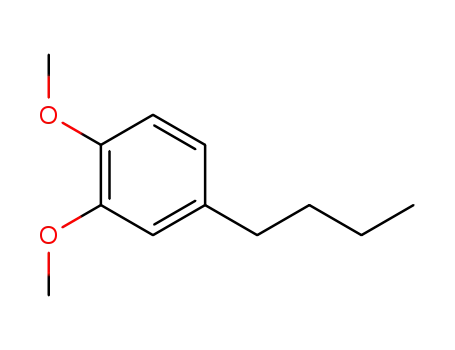
1,2-dimethoxy-4-butylbenzene

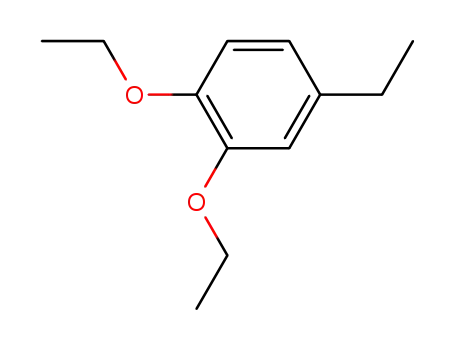
1,2-diethoxy-4-ethyl-benzene

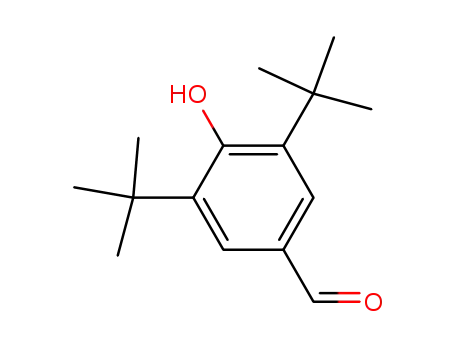
3,5-di-t-butyl-4-hydroxybenzaldehyde
| Conditions | Yield |
|---|---|
|
With
ortho-tungstic acid;
at 300 ℃;
for 6h;
Autoclave;
|

ethanol


2-methoxy-phenol

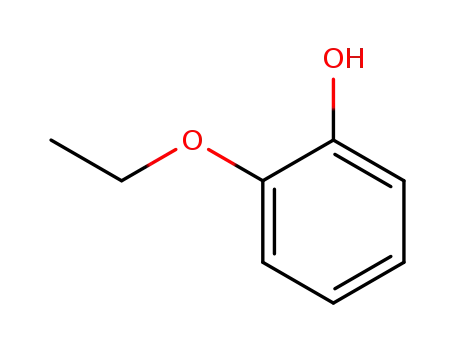
2-Ethoxyphenol


3,5-Di-tert-butylcatechol


2,4-diisopropylphenol


2,6-diisopropylphenol


2,4,6-triisopropylphenol


2,6-di-tert-butyl-4-ethylphenol


2,6-di-tert-butyl-4-hydroxyphenol


2,6-di-tert-butyl-4-methoxymethylene-phenol


3,5-Di-tert-butylphenol


2,4-dimethyl-6-tert-butylphenol


2-(2',5'-dimethoxyphenyl)propionaldehyde


2,6-di-tert-butyl-4-sec-butylphenol


1,2-diethoxybenzene


1,4-dimethoxy-2-tert-butylbenzene


1-ethoxy-4-methoxybenzene


2,5-di(tert-amyl)-1,4-hydroquinone


2,4-di-tert-amylphenol


2,5-diethyl phenol


4-ethoxy-3-methoxytoluene


1,2-dimethoxy-4-butylbenzene


1,2-diethoxy-4-ethyl-benzene


3,5-di-t-butyl-4-hydroxybenzaldehyde
| Conditions | Yield |
|---|---|
|
With
tungsten(VI) oxide;
at 300 ℃;
for 6h;
Reagent/catalyst;
Autoclave;
|

2,4-diisopropylphenol

ethanol

2-methoxy-phenol
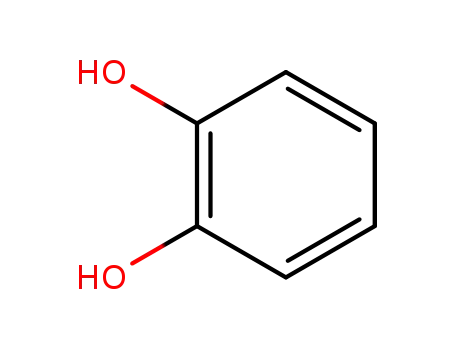
benzene-1,2-diol
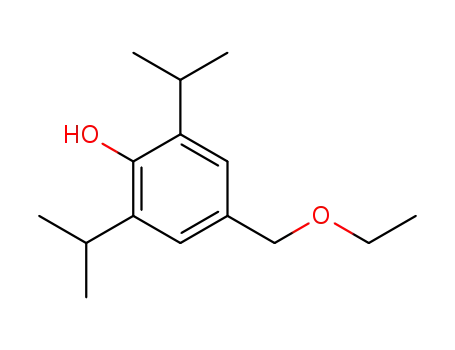
4-ethoxymethyl-2,6-diisopropyl-phenol
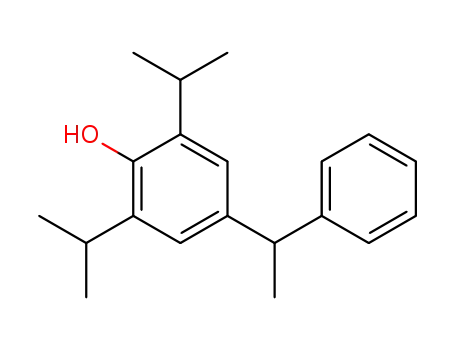
2,6-diisopropyl-4-(1-phenyl-ethyl)-phenol
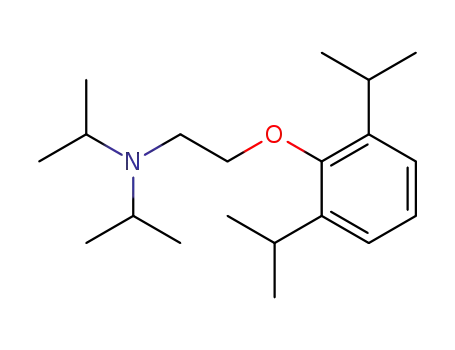
[2-(2,6-diisopropyl-phenoxy)-ethyl]-diisopropyl-amine
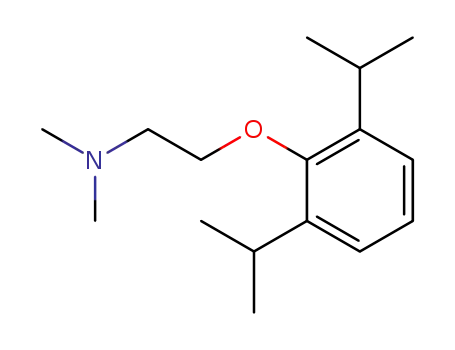
[2-(2,6-diisopropyl-phenoxy)-ethyl]-dimethyl-amine