
Antioxidant BHT 264
CAS:128-37-0
Purity:99%
Contact Now
We will contact you as soon as possible
Your Location:Home >Products >Intermediates >121-57-3
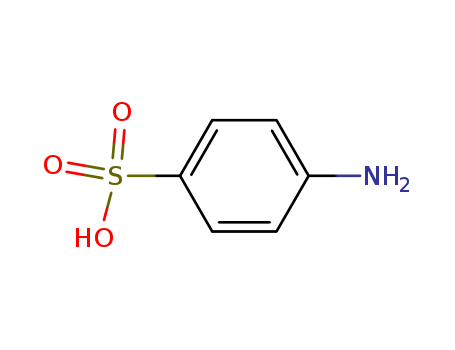

Product Details
|
Chemical Composition and Structure |
Sulfanilic acid typically exists as a white to off-white crystalline solid with a slight odor. It is soluble in water and has a melting point around 288-290°C. Sulfanilic acid has the chemical formula C6H7NO3S, with a molecular weight of 173.19 g/mol. It contains an amino group (NH2) and a sulfonic acid group (SO3H) attached to a benzene ring. |
|
Uses and Mechanism of Action |
In chemistry, sulfanilic acid serves as a precursor in the synthesis of various compounds, including dyes, pharmaceuticals, and coordination polymers.[4] |
|
History and Development |
Sulfanilic acid has been used industrially for many years, particularly in the dye industry. Its synthesis from readily available starting materials has made it a versatile compound for various applications. |
|
Analysis Method |
The purity and identity of sulfanilic acid can be determined using various spectroscopic techniques such as FTIR, Raman spectroscopy, UV-Vis spectroscopy, and ESR spectroscopy. These methods help ensure product quality and consistency, as well as provide insights into its molecular structure and properties. |
|
Production Methods |
Sulfanilic acid is primarily synthesized from aniline and concentrated sulfuric acid. It can also be obtained as a byproduct in the production of aniline and is widely available commercially.[3] |
|
Synthesize |
Sulfanilic acid can be used for the determination of nitrite. Biochemical Research. Organic Synthesis. Sulfanilic acid is an important intermediate of sulfonamide drugs. This product has strong antibacterial effect on hemolytic streptococcus, meningitis and coccus, but due to poor efficacy and high toxicity, it is rarely used for oral administration. It is also rarely used. It is used as an intermediate for the synthesis of other sulfonamides, and is also used as a raw material for the synthesis of agricultural "Huangcaoling" abroad. Sulfanilic acid can also be used as an analytical reagent, such as a reagent for the photometric determination of nitrite and sodium nitroferricyanide. For biochemical research, organic synthesis and pharmaceutical industry. Sulfanilic acid is the main raw material for synthesizing sulfonamides. In addition to preparing crystalline sulfonamides for external anti-inflammatory, it can also synthesize other sulfonamides such as sulfamidine, sulfamethoxazine, and sulfamethazine. |
|
Definition |
ChEBI: An aminobenzenesulfonic acid that is aniline sulfonated at the para-position. |
|
Application |
As the compound readily form diazo compounds, it is used to make dyes and sulpha drugs . This property is also used for the quantitative analysis of nitrate and nitrite ions by diazonium coupling reaction with N-(1-Naphthyl) ethylene diamine , resulting in an azo dye, and the concentration of nitrate or nitrite ions were deduced from the color intensity of the resulting red solution by colorimetry. It is also used as a standard in combustion analysis. |
|
General Description |
White powder with faint purple tinge. Grayish-white flat crystals. Becomes anhydrous at around 212°F. Low toxicity (used medicinally). |
|
Air & Water Reactions |
Insoluble in water. |
|
Reactivity Profile |
Sulfanilic acid is an amino acid. Reacts weakly with both acids and bases. |
|
Fire Hazard |
Flash point data for Sulfanilic acid are not available, however Sulfanilic acid is probably combustible. |
|
Synthesis |
Sulfanilic acid can be produced by sulfonation of aniline. |
|
Purification Methods |
Crystallise the acid (as dihydrate) from boiling water. Dry it at 105o for 2-3hours, then over 90% H2SO4 in a vacuum desiccator. The S-benzylisothiuronium salt has m 187o (from aqueous EtOH). [Beilstein 14 IV 2655.] |
InChI:InChI=1/C6H7NO3S/c7-5-1-3-6(4-2-5)11(8,9)10/h1-4H,7H2,(H,8,9,10)/p-1
In the photolysis of sodium metanilate (...
Activated carbon products modified with ...
Abstract: Toluene disocyanate (TDI) was ...
Polyaniline (PANI) and Polyaniline/Nicke...
Abstract: A novel magnetically recyclabl...
This study focused on examining the gene...
The reduction of azo dyes by zero-valent...
-
Cationic dye (methylene blue) and anioni...
In alkaline media, well-characterized ge...
Photo-decomposition of sulfamethazine (S...
The present study reports the synthesis ...
-
Excitation of TiO2 for visible light abs...
The scale-up synthesis of nZVI (zero-val...
The invention discloses a preparation me...
Cornforth and Corey-Suggs reagents Pyrid...
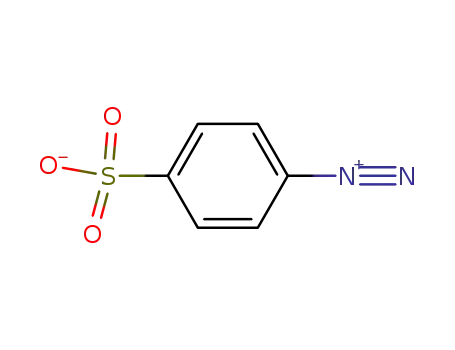
4-diazobenzenesulfonic acid

water

aniline hydrochloride

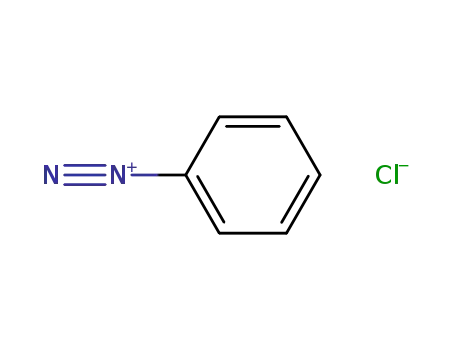
benzene diazonium chloride

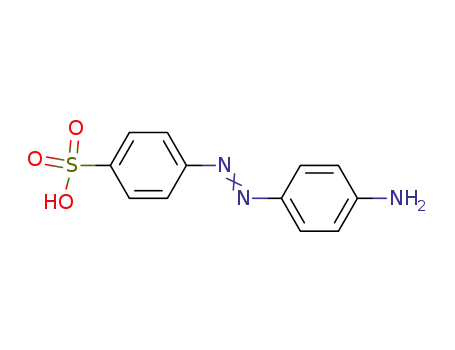
4'-aminoazobenzene-4-sulfonic acid

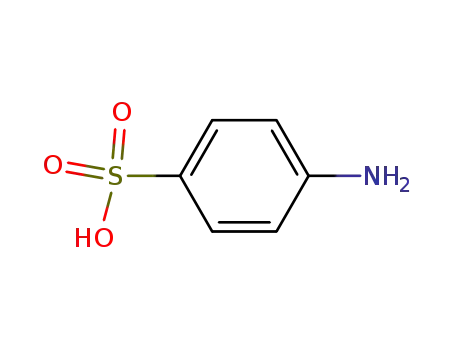
4-aminobenzene sulfonic acid
| Conditions | Yield |
|---|---|
|
|
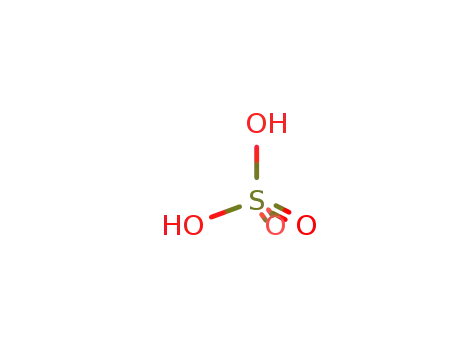
sulfuric acid

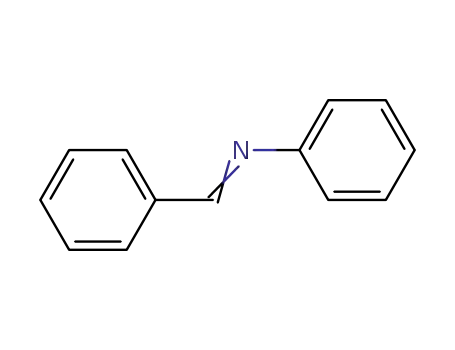
benzylidene phenylamine

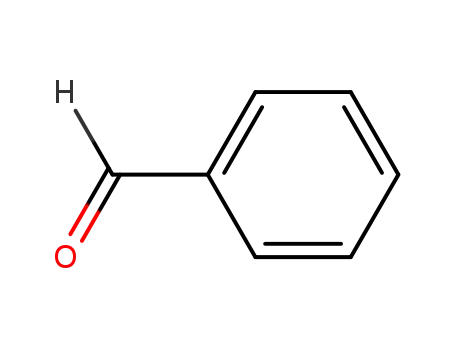
benzaldehyde


4-aminobenzene sulfonic acid
| Conditions | Yield |
|---|---|
|
und Erhitzen der mit Wasser verd.Loesung;
|
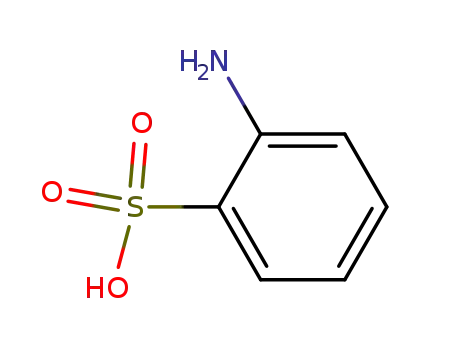
2-amino-1-benzenesulfonic acid
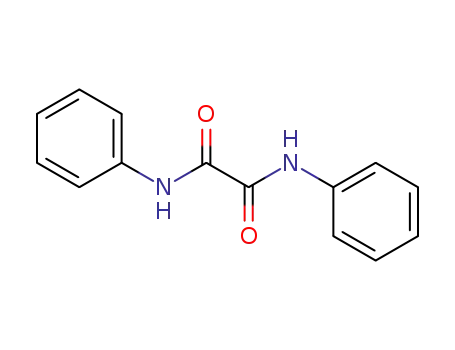
N,N'-Diphenyloxamid
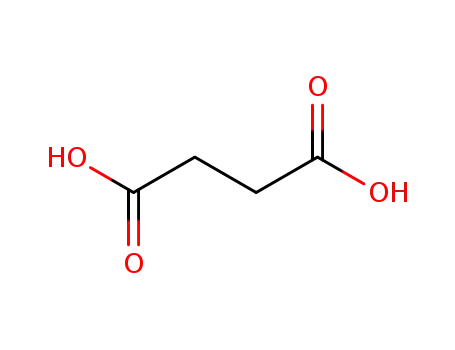
succinic acid
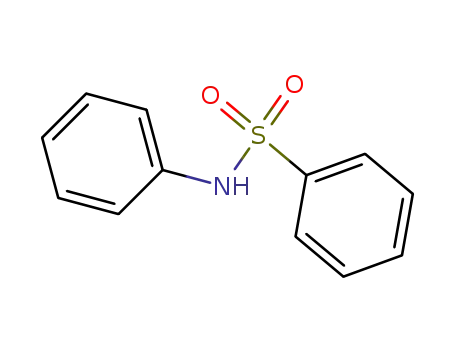
N-phenylbenzenesulfonamide
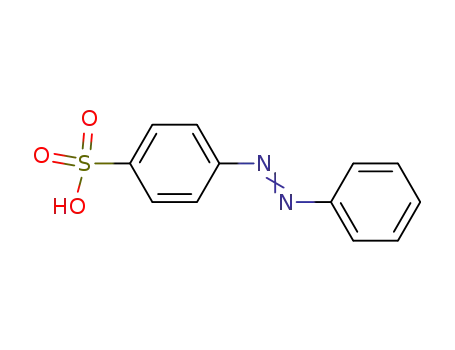
4-phenylazo-benzenesulfonic acid
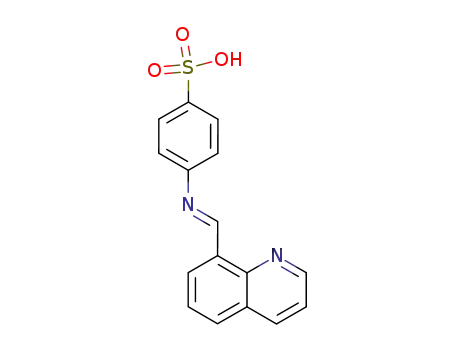
N-[8]quinolylmethylene-sulfanilic acid
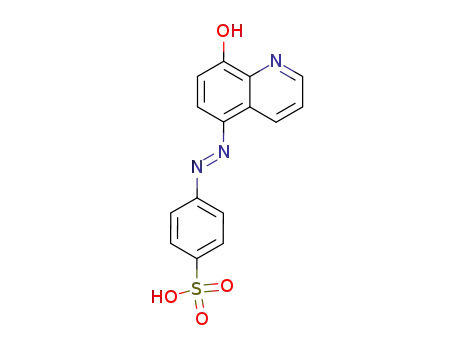
(E)-4-((8-hydroxyquinolin-5-yl)diazenyl)benzenesulfonic acid
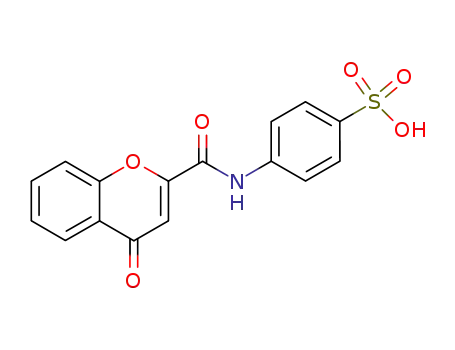
4-(4-oxo-4H-chromene-2-carbonylamino)-benzenesulfonic acid