
Antioxidant BHT 264
CAS:128-37-0
Purity:99%
Contact Now
We will contact you as soon as possible
Your Location:Home >Products >Industrial Chemicals >1879-09-0
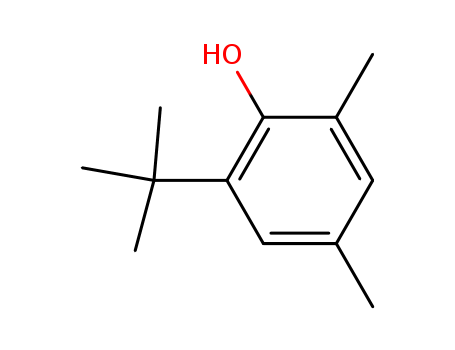

Product Details
|
Air & Water Reactions |
Insoluble in water. |
|
Reactivity Profile |
Phenols, such as 2-(tert-Butyl)-4,6-dimethylphenol, do not behave as organic alcohols, as one might guess from the presence of a hydroxyl (-OH) group in their structure. Instead, they react as weak organic acids. Phenols and cresols are much weaker as acids than common carboxylic acids (phenol has Ka = 1.3 x 10^[-10]). These materials are incompatible with strong reducing substances such as hydrides, nitrides, alkali metals, and sulfides. Flammable gas (H2) is often generated, and the heat of the reaction may ignite the gas. Heat is also generated by the acid-base reaction between phenols and bases. Such heating may initiate polymerization of the organic compound. Phenols are sulfonated very readily (for example, by concentrated sulfuric acid at room temperature). The reactions generate heat. Phenols are also nitrated very rapidly, even by dilute nitric acid. Nitrated phenols often explode when heated. Many of them form metal salts that tend toward detonation by rather mild shock. |
|
Health Hazard |
TOXIC; inhalation, ingestion or skin contact with material may cause severe injury or death. Contact with molten substance may cause severe burns to skin and eyes. Avoid any skin contact. Effects of contact or inhalation may be delayed. Fire may produce irritating, corrosive and/or toxic gases. Runoff from fire control or dilution water may be corrosive and/or toxic and cause pollution. |
|
Fire Hazard |
Non-combustible, substance itself does not burn but may decompose upon heating to produce corrosive and/or toxic fumes. Some are oxidizers and may ignite combustibles (wood, paper, oil, clothing, etc.). Contact with metals may evolve flammable hydrogen gas. Containers may explode when heated. |
|
General Description |
A yellow liquid with a phenolic odor. Insoluble in water and about the same density as water. Exposure to skin, eyes or mucous membranes may cause severe burns. |
InChI:InChI=1/C15H16Cl3N3O2.C15H24O/c1-2-4-20(15(22)21-5-3-19-10-21)6-7-23-14-12(17)8-11(16)9-13(14)18;1-10-8-11(14(2,3)4)13(16)12(9-10)15(5,6)7/h3,5,8-10H,2,4,6-7H2,1H3;8-9,16H,1-7H3
-
The microwave-accelerated Claisen rearra...
Abstract: To support the industrial desi...
The conversion of guaiacol is examined a...
The invention provides a phenol o-positi...
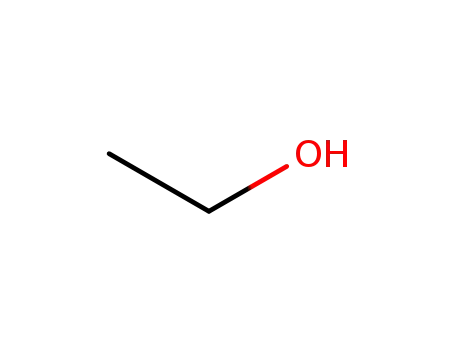
ethanol

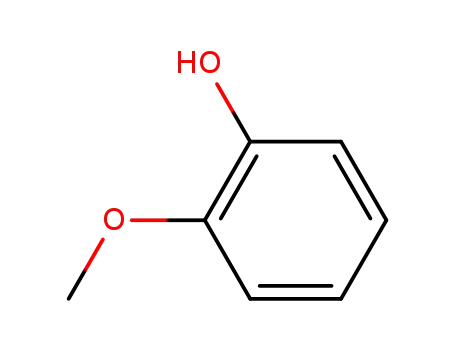
2-methoxy-phenol

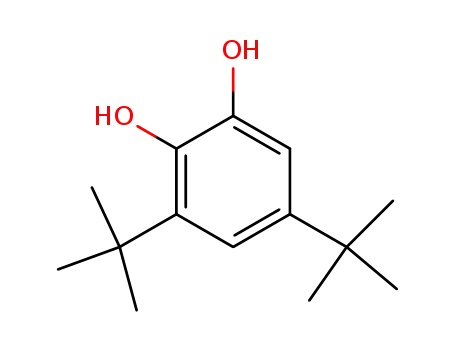
3,5-Di-tert-butylcatechol

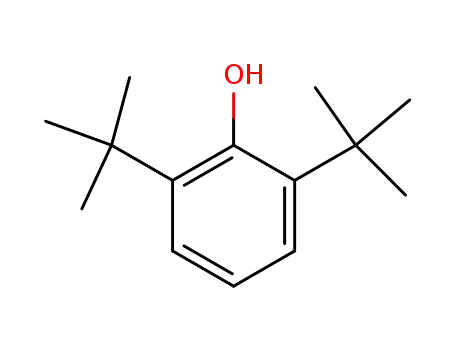
2,6-di-tert-butylphenol

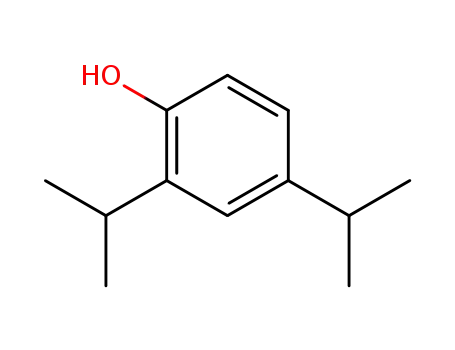
2,4-diisopropylphenol

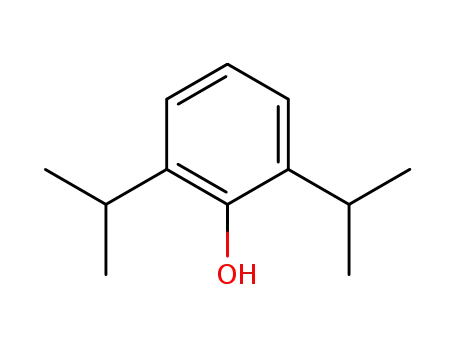
2,6-diisopropylphenol

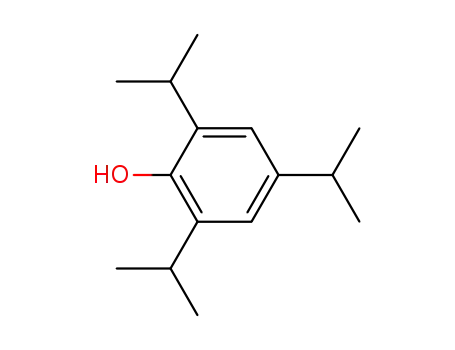
2,4,6-triisopropylphenol

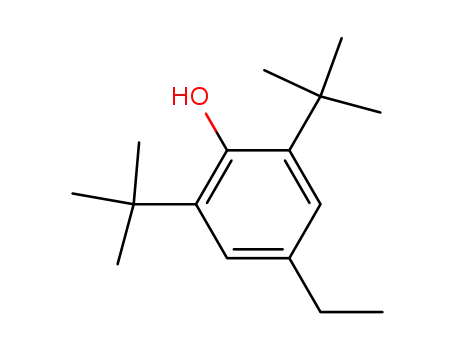
2,6-di-tert-butyl-4-ethylphenol

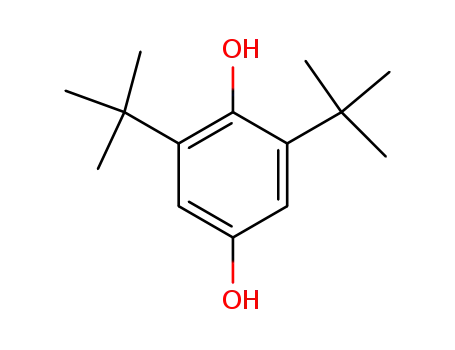
2,6-di-tert-butyl-4-hydroxyphenol

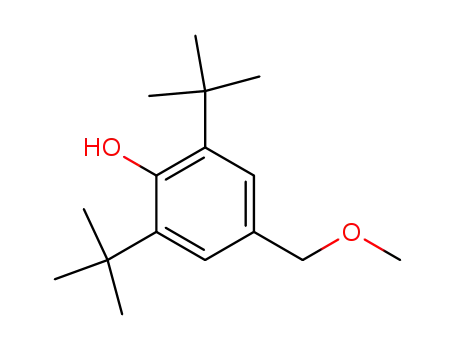
2,6-di-tert-butyl-4-methoxymethylene-phenol

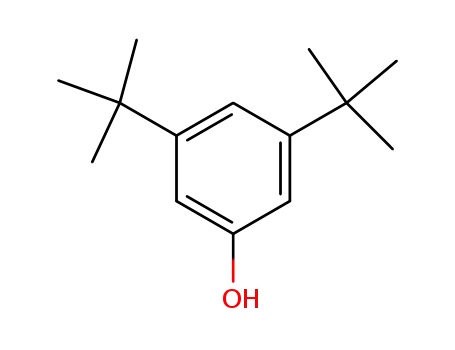
3,5-Di-tert-butylphenol

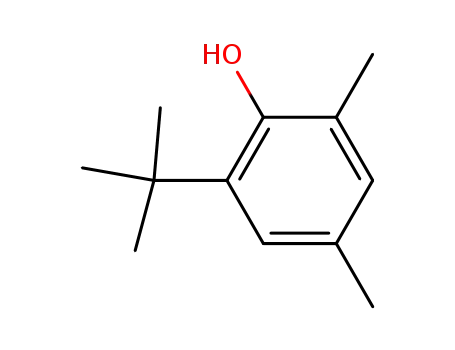
2,4-dimethyl-6-tert-butylphenol

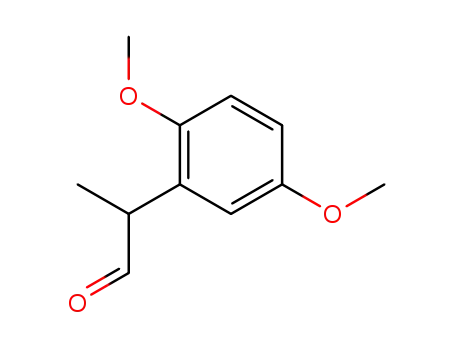
2-(2',5'-dimethoxyphenyl)propionaldehyde

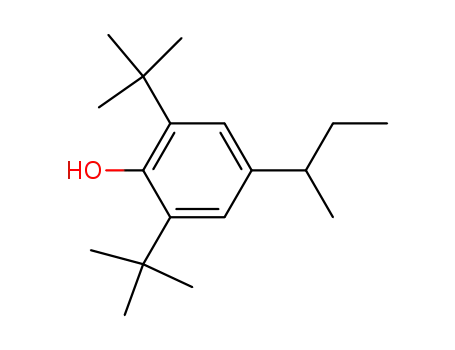
2,6-di-tert-butyl-4-sec-butylphenol

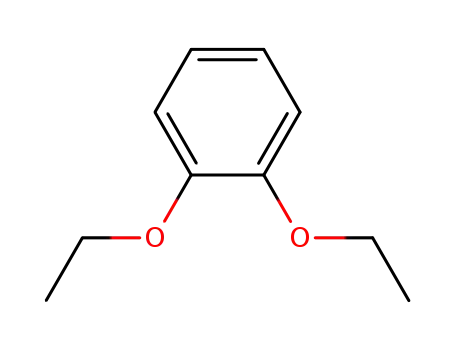
1,2-diethoxybenzene

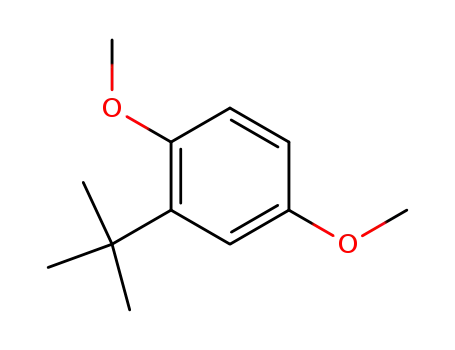
1,4-dimethoxy-2-tert-butylbenzene

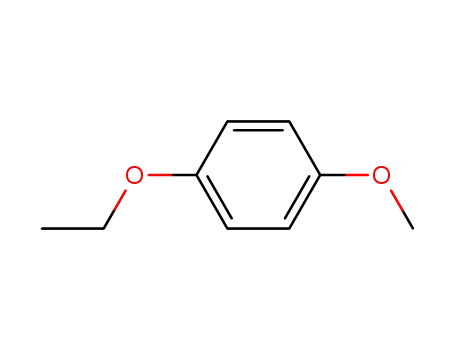
1-ethoxy-4-methoxybenzene

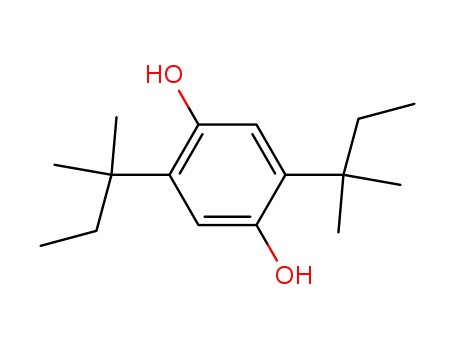
2,5-di(tert-amyl)-1,4-hydroquinone

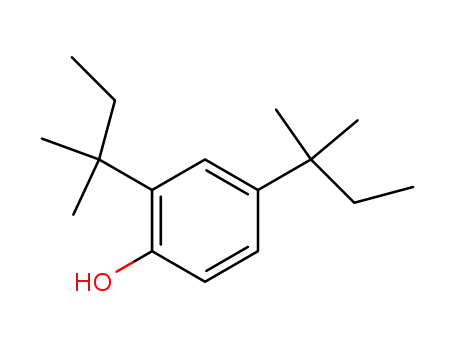
2,4-di-tert-amylphenol

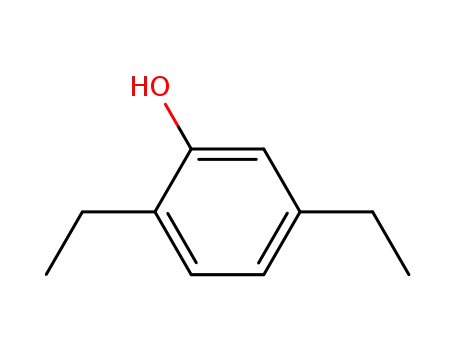
2,5-diethyl phenol

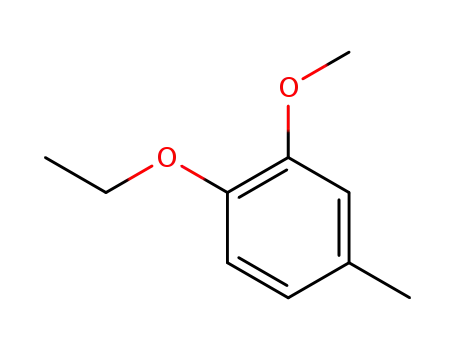
4-ethoxy-3-methoxytoluene

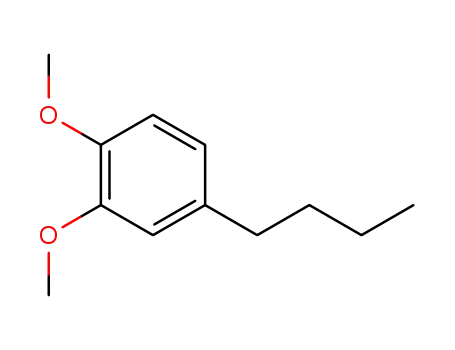
1,2-dimethoxy-4-butylbenzene

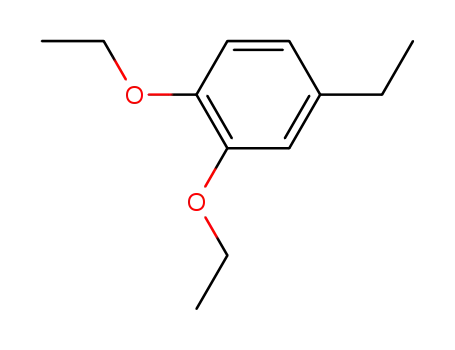
1,2-diethoxy-4-ethyl-benzene

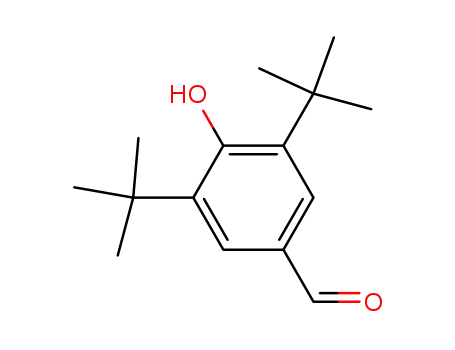
3,5-di-t-butyl-4-hydroxybenzaldehyde
| Conditions | Yield |
|---|---|
|
With
ortho-tungstic acid;
at 300 ℃;
for 6h;
Autoclave;
|

ethanol


2-methoxy-phenol

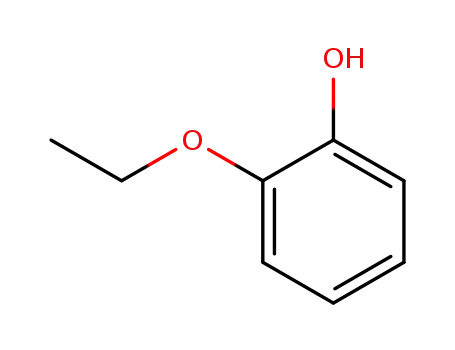
2-Ethoxyphenol


3,5-Di-tert-butylcatechol


2,4-diisopropylphenol


2,6-diisopropylphenol


2,4,6-triisopropylphenol


2,6-di-tert-butyl-4-ethylphenol


2,6-di-tert-butyl-4-hydroxyphenol


2,6-di-tert-butyl-4-methoxymethylene-phenol


3,5-Di-tert-butylphenol


2,4-dimethyl-6-tert-butylphenol


2-(2',5'-dimethoxyphenyl)propionaldehyde


2,6-di-tert-butyl-4-sec-butylphenol


1,2-diethoxybenzene


1,4-dimethoxy-2-tert-butylbenzene


1-ethoxy-4-methoxybenzene


2,5-di(tert-amyl)-1,4-hydroquinone


2,4-di-tert-amylphenol


2,5-diethyl phenol


4-ethoxy-3-methoxytoluene


1,2-dimethoxy-4-butylbenzene


1,2-diethoxy-4-ethyl-benzene


3,5-di-t-butyl-4-hydroxybenzaldehyde
| Conditions | Yield |
|---|---|
|
With
tungsten(VI) oxide;
at 300 ℃;
for 6h;
Reagent/catalyst;
Autoclave;
|
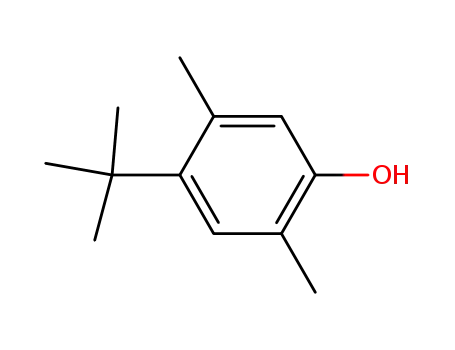
2,5-dimethyl-4-(1,1-dimethylethyl)-phenol
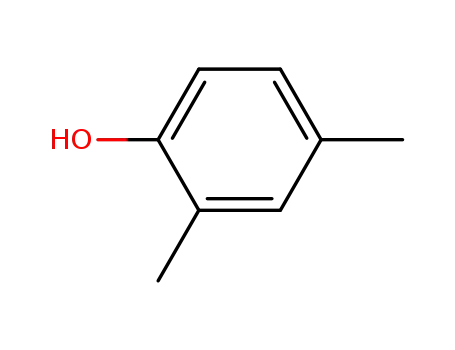
2,4-Xylenol
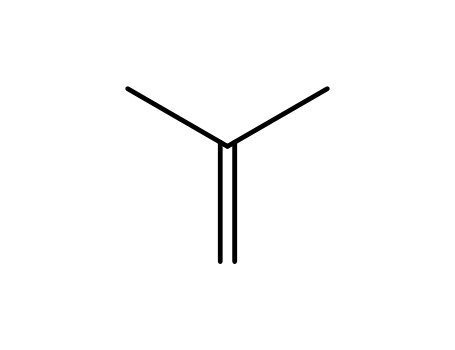
isobutene
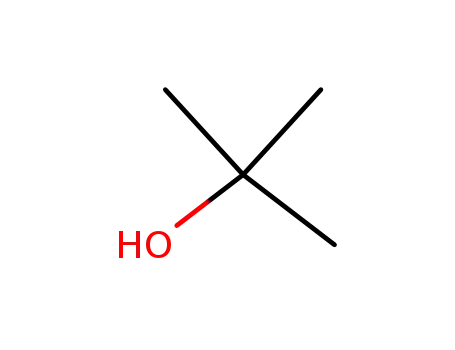
tert-butyl alcohol
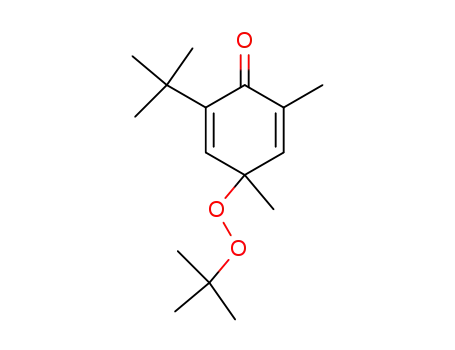
2-t-butyl-4-t-butylperoxy-4,6-dimethyl-2,5-cyclohexadien-1-one
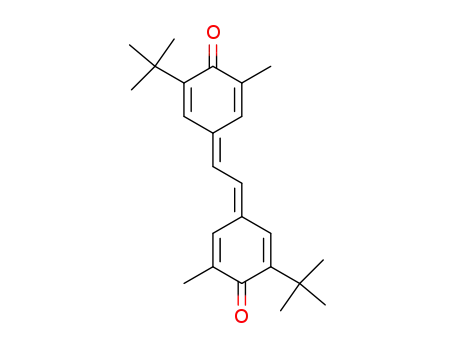
2,2'-di-tert-butyl-6,6'-dimethyl-4,4'-ethanediylidene-bis-cyclohexa-2,5-dienone
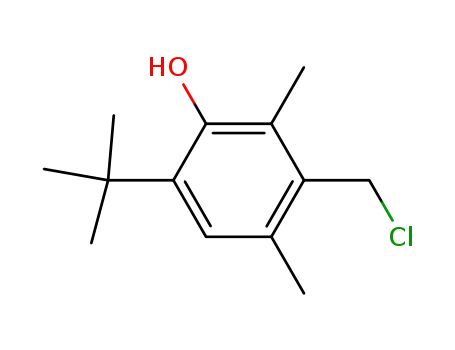
2,6-di-methyl-4-tert-butyl-3-hydroxybenzyl chloride

2,4-Xylenol