
Antioxidant BHT 264
CAS:128-37-0
Purity:99%
Contact Now
We will contact you as soon as possible
Your Location:Home >Products >Intermediates >70-55-3

Product Details
|
Role and purpose |
P-toluene sulfonamide is a kind of excellent solid plasticizer used for thermoset plastic. It is suitable for being applied to phenolic resin, melamine resin, urea-formaldehyde resins and polyamide resins. Combination with a small amount can improve workability and make the solidification be uniform, and thus endowing the products with a good gloss. However, P-toluene sulfonamide has no softening effect as the liquid plasticizer, and is incompatible with polyvinyl chloride and vinyl chloride copolymer and is partially compatible with cellulose acetate, cellulose acetate butyrate and cellulose nitrate. p-Toluene sulfonamide has a low toxicity. It has been approved by the US Food and Drug Administration for being applied into adhesives as food packaging materials. p-Toluene sulfonamide is briefly called TSH and is commonly used for nickel plating. It can improve the coating structure by the adsorption on the electrode so that it has some degree of surface glossing effect; it is generally not strictly limited for the added amount of brighteners lax; moreover it also has very light effects on the coating characteristics. However, it can only give semi-bright coatings, and the surface gloss is related to the polishing quality of the matrix before plating. Currently, the brightener agents of nickel plating used is mostly some kinds of organic compounds. And the used amount of these organic compound, although are very little, but with a significant effect. In addition to brightening the coating, it can also largely determine the mechanical properties and chemical properties of the coating. But it must be realized that, since the bright nickel plating solution has been added to the organic brightener, it naturally makes the nickel plating layer contain a relative big amount of organic sulfur and other impurities, thus inevitably enlarging the internal stress, so its mechanical properties is a bitter lower than the conventional nickel-plate, and is prone to crack layer when the plating gets stress; its antirust ability is also worse than the dark nickel. Thus, a single layer of bright nickel is unable to improve the corrosion resistance and mechanical properties of the nickel layer; it must need to form a bi-layer and multilayer nickel or chromium to form a multilayer combination of decorative protective coatings. When toluene sulfonamide is heated to 105 °C, it will be decomposed and there will be nitrogen released (gas evolution 130 mL/g); p-toluene sulfonamide foaming agents can give a fine foaming with small product shrinkage. It also has a high tear strength and good stability. Thereby, it has a wide range of applications. The above information is edited by the lookchem of Dai xiongfeng. |
|
Production methods |
It is obtained by the reaction between toluenesulfonyl chloride and ammonia. First, add part of HN3 water into the reaction pot, stirring for adding p-toluenesulfonyl chloride with the temperature rising naturally to above 50 °C; after the temperature has dropped, add the remaining ammonia and have reaction for 0.5 h at 85~90 °C. Stop the reaction when the pH reaches 8 to 9. Cool to 20 °C, filter, and wash the filter cake with water to obtain the crude. Then further go through bleaching via activated carbon, alkali dissolution, acid precipitation, rejection filter, drying to get products. |
|
Synthesis Reference(s) |
The Journal of Organic Chemistry, 47, p. 4327, 1982 DOI: 10.1021/jo00143a031Synthetic Communications, 20, p. 293, 1990 DOI: 10.1080/00397919008052297Synthesis, p. 1031, 1986 DOI: 10.1055/s-1986-31862 |
|
Purification Methods |
Crystallise the amide from hot water, then from EtOH or Et2O/pet ether. [Beilstein 11 H 104, 11 IV 376.] |
|
Definition |
ChEBI: A sulfonamide that is benzenesulfonamide bearing a methyl group at position 4. |
|
General Description |
p-Toluenesulfonamide undergoes FeCl3-catalyzed direct substitution reaction with benzylic and allylic alcohols.It is employed as nucleophile in tetrabutylammonium fluoride (TBAF) catalyzed vinyl aziridine opening reaction. |
InChI:InChI=1/C7H9NO2S/c1-6-2-4-7(5-3-6)11(8,9)10/h2-5H,1H3,(H2,8,9,10)
The development of nanoparticle-based bi...
Kinetics of oxidation of L-threonine in ...
Rh(III)-catalyzed C-H activation/annulat...
The kinetics of osmium(VIII)-catalysed o...
N-[1-4-Methoxyphenyl-2-phenyl-2,2-dichlo...
S-(9-Fluorenylidene)-N-(p-tolylsulfonyl)...
We have synthesized both the 4 and 5 tau...
A divergent synthetic approach to polycy...
The disulphide dication of 1,5-dithiacyc...
The reactions of 3,4-di-t-butyl-1-[(p-to...
The N-tosylaziridine 4 of (+)-2-carene 1...
The syntheses of new sulphonamide deriva...
Hydrogen sulfide (H2S) is an important s...
A novel method has been developed to syn...
Transition metal-catalyzed diamination b...
Oxidation is a vital step of drug metabo...
Ecto-nucleotide pyrophosphatases/phospho...
Abstract: Sulfonamides is an important c...
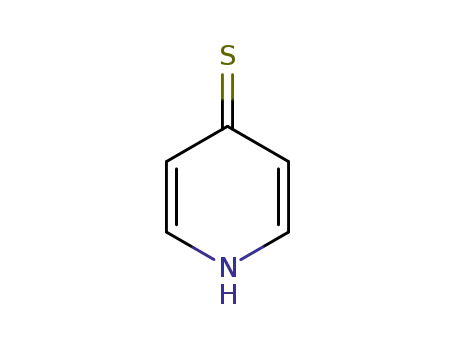
4-pyridinethione

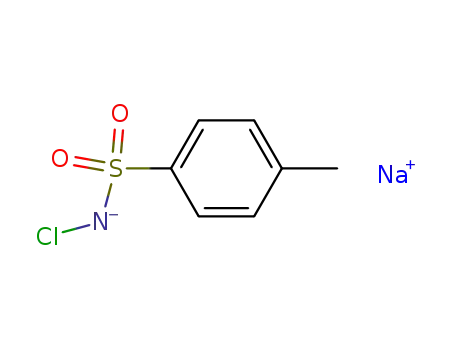
chloroamine-T

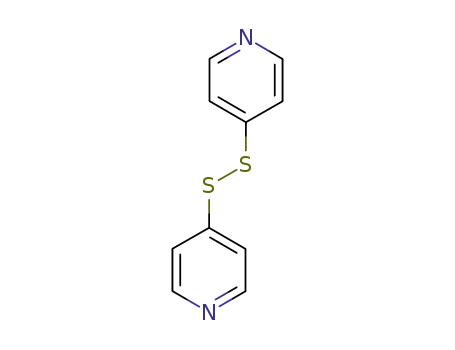
dipyridin-4-yl disulfide

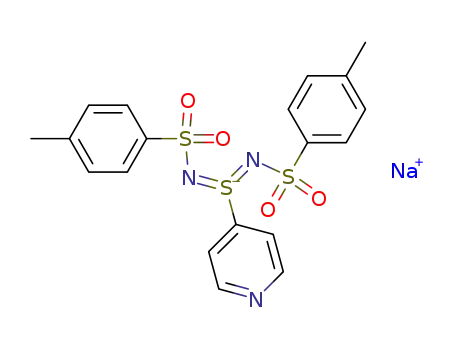
Natrium-S-(4-pyridyl)-N,N'-bis(tosyl)sulfodiimidat

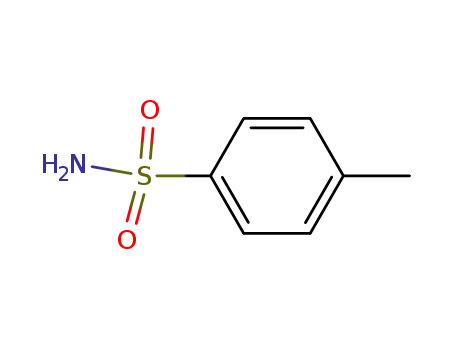
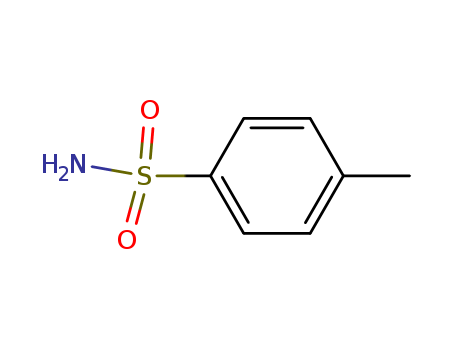
toluene-4-sulfonamide
| Conditions | Yield |
|---|---|
|
In
ethanol;
at 0 ℃;
for 3h;
|
36% |
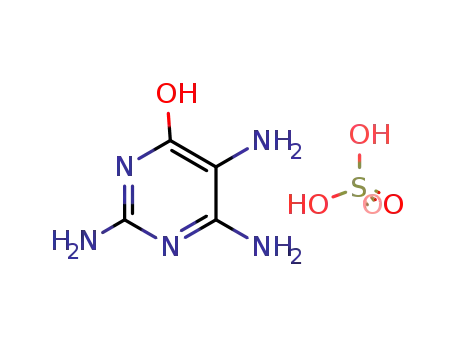
2,4,5-triamino-6-hydroxypyrimidine sulfate

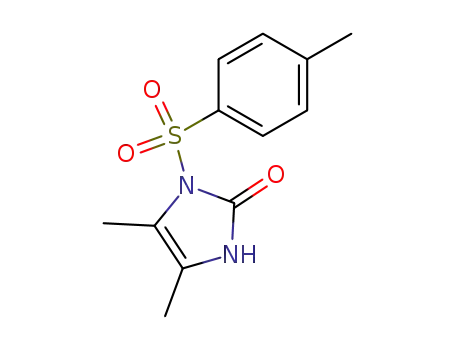
1-tosyl-4,5-dimethylimidazolin-2-one

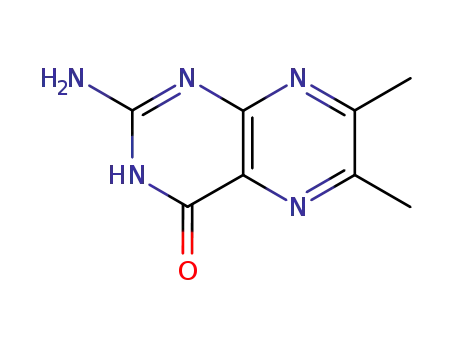
6,7-Dimethylpterin


toluene-4-sulfonamide
| Conditions | Yield |
|---|---|
|
With
piperidine;
In
water;
for 140h;
|
67% |
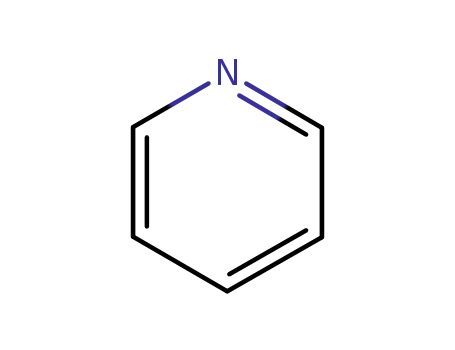
pyridine
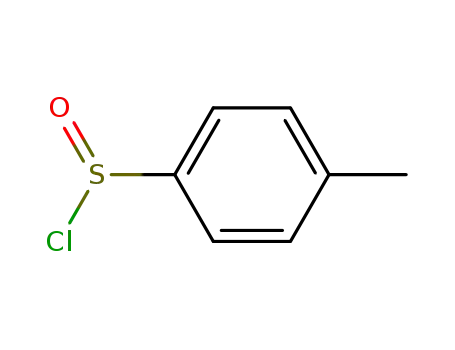
4-methylbenzenesulfinyl chloride
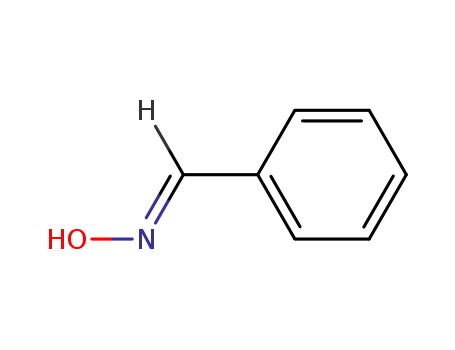
syn-benzaldehyde oxime
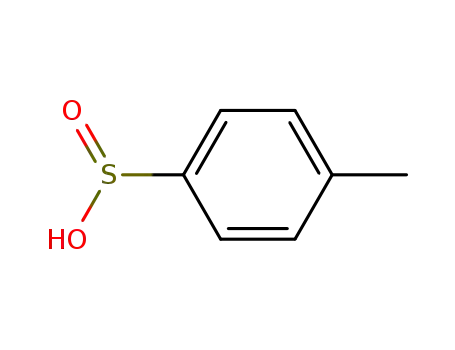
p-toluene sulfinic acid
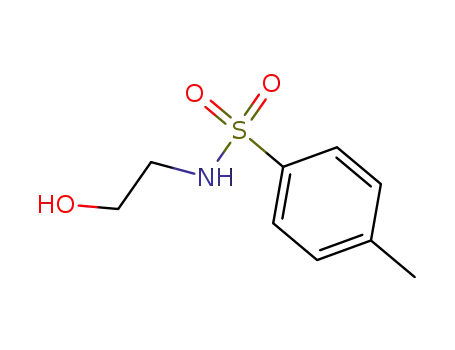
N-(2-hydroxy-ethyl)-4-methyl-benzenesulfonamide
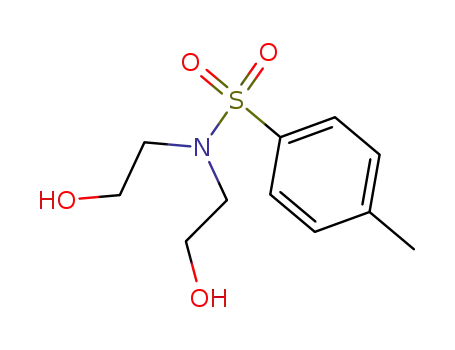
N-tosyldiethanolamine
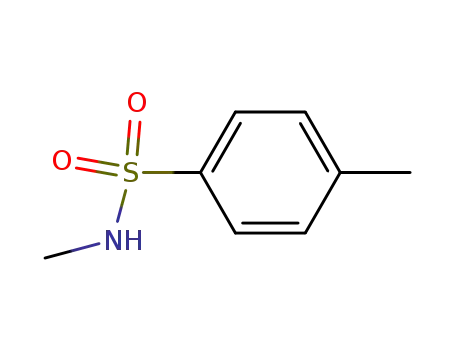
N-methyl-p-toluenesulfonylamide
1-[1,4-dioxo-3-(toluene-4-sulfonylamino)-1,4-dihydro-[2]naphthyl]-pyridinium betaine