
Antioxidant BHT 264
CAS:128-37-0
Purity:99%
Contact Now
We will contact you as soon as possible
Your Location:Home >Products >Pharmaceutical API >154229-18-2
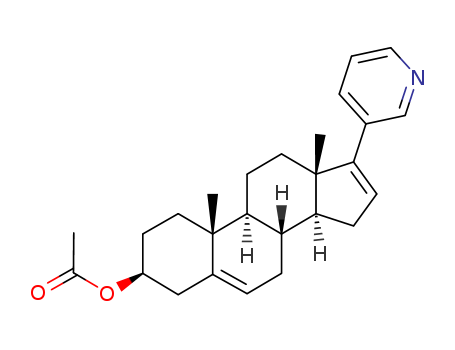

Product Details
|
Biochem/physiol Actions |
Abiraterone acetate is a prodrug of abiraterone, which is a potent, selective, and orally bioavailable inhibitor of CYP17A1 (CYP450c17), an enzyme that catalyzes two key serial reactions (17α hydroxylase and 17,20 lyase) in androgen and estrogen biosynthesis resulting in the formation of DHEA and androstenedione, which may ultimately be metabolized into testosterone. CYP17 is the key enzyme for androgen biosynthesis in both the testes and adrenals, so its inhibition should stop the production of androgens in both places. Abiraterone acetate is used for the treatment of metastatic castration-resistant prostate cancer. Abiraterone acetate possesses significant antitumor activity in post-docetaxel patients with CRPC (castration-resistant prostate cancer). It is highly essential for androgen biosynthesis in the testes, adrenal glands, and prostate tissue. |
|
Synthesis |
The most convenient synthesis for scale-up will be highlighted from two published syntheses. Commercially available androstenolone 1 was acylated with acetic anhydride in the presence of boron trifluoride-diethyl etherate to give a near quantitative yield of acetate 2. The conversion of ketone 2 to vinyl triflate 3 involved careful selection of base to prevent elimination of the acetate group. To this extent, subjection of 2 to triflic anhydride in dichloromethane at ambient temperature followed by slow addition of triethylamine minimized undesired side products and delivered triflate 3 in 60% isolated yield. Subsequent Suzuki coupling with diethylborane 4 under standard conditions ultimately furnished abiraterone acetate (I) in 75% yield. |
|
Drug interactions |
Potentially hazardous interactions with other drugs Antibacterials: concentration possibly reduced by rifabutin and rifampicin - avoid. Antidepressants: concentration possibly reduced by St John’s wort - avoid. Antiepileptics: concentration possibly reduced by carbamazepine, fosphenytoin, phenobarbital, phenytoin and primidone - avoid. |
|
Metabolism |
Abiraterone acetate is hydrolysed to abiraterone, which then undergoes metabolism including sulphation, hydroxylation and oxidation mainly in the liver to form inactive metabolites. About 88% of a dose is excreted in the faeces, of which about 55% is unchanged abiraterone acetate and about 22% is abiraterone; about 5% of a dose is excreted in the urine. |
|
Definition |
ChEBI: A sterol ester obtained by formal condensation of the 3-hydroxy group of abiraterone with the carboxy group of acetic acid. A prodrug that is converted in vivo to abiraterone. Used for treatment of metastatic castrate-resistant prostate cance . |
|
Brand name |
Zytiga |
InChI:InChI=1/C26H33NO2/c1-17(28)29-20-10-12-25(2)19(15-20)6-7-21-23-9-8-22(18-5-4-14-27-16-18)26(23,3)13-11-24(21)25/h4-6,8,14,16,20-21,23-24H,7,9-13,15H2,1-3H3/t20-,21?,23?,24?,25-,26+/m0/s1
An improved procedure for the preparatio...
Abiraterone acetate is used for the trea...
The invention particularly relates to ap...
Substantial evidence underscores the cli...
The invention provides a method for prep...
The invention provides an abiraterone ac...
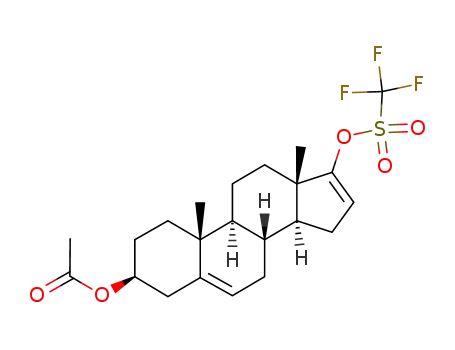
3β-acetoxyandrosta-5,16-dien-17-yl trifluoromethanesulphonate

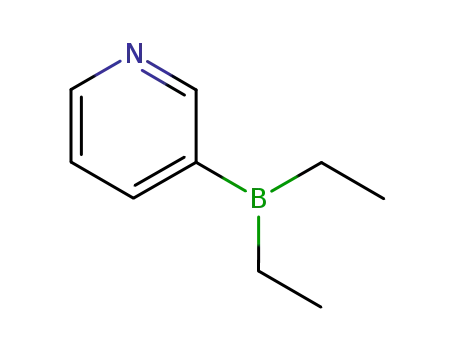
3-Diethylboranylpyridine

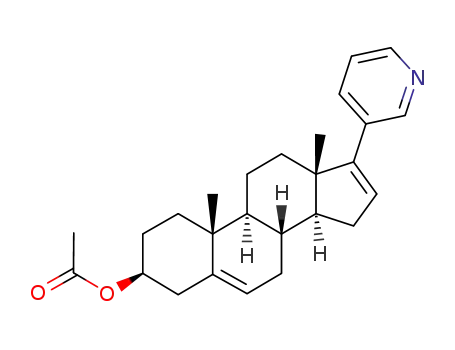
abiraterone acetate
| Conditions | Yield |
|---|---|
|
With
bis-triphenylphosphine-palladium(II) chloride; sodium carbonate;
In
tetrahydrofuran;
at 65 ℃;
for 4h;
Reflux;
|
95% |
|
With
bis-triphenylphosphine-palladium(II) chloride; sodium carbonate;
In
tetrahydrofuran; water;
for 1h;
Reflux;
|
95% |
|
With
bis-triphenylphosphine-palladium(II) chloride; sodium hydrogencarbonate;
In
tetrahydrofuran; water;
at 60 ℃;
for 1h;
|
94% |
|
With
bis-triphenylphosphine-palladium(II) chloride; sodium carbonate;
In
tetrahydrofuran; water;
for 18h;
Inert atmosphere;
Reflux;
|
90% |
|
With
bis(triphenylphosphine)palladium(II)-chloride; sodium carbonate;
In
tetrahydrofuran;
at 80 ℃;
for 1h;
|
84% |
|
With
bis-triphenylphosphine-palladium(II) chloride; sodium hydrogencarbonate;
In
tetrahydrofuran; water;
at 60 ℃;
for 23h;
Inert atmosphere;
|
77% |
|
With
bis-triphenylphosphine-palladium(II) chloride; sodium carbonate;
In
tetrahydrofuran; water;
for 1h;
Inert atmosphere;
Reflux;
|
|
|
With
bis-triphenylphosphine-palladium(II) chloride; sodium carbonate;
In
2-methyltetrahydrofuran; water;
at 20 - 80 ℃;
for 2.15h;
Inert atmosphere;
|
|
|
With
bis-triphenylphosphine-palladium(II) chloride; sodium carbonate;
In
tetrahydrofuran; water;
at 66 ℃;
for 3.5h;
|
15.68 g |
|
With
bis-triphenylphosphine-palladium(II) chloride; sodium carbonate;
In
tetrahydrofuran; water;
at 25 - 80 ℃;
for 6h;
Inert atmosphere;
|
|
|
With
bis-triphenylphosphine-palladium(II) chloride; sodium carbonate;
In
tetrahydrofuran; water;
at 20 ℃;
for 1.5h;
Temperature;
Concentration;
Reflux;
Large scale;
|
65 kg |
|
3β-acetoxyandrosta-5,16-dien-17-yl trifluoromethanesulphonate; 3-Diethylboranylpyridine;
With
bis-triphenylphosphine-palladium(II) chloride;
In
tetrahydrofuran;
at 20 ℃;
for 0.0833333h;
Inert atmosphere;
With
sodium carbonate;
In
tetrahydrofuran; water;
Inert atmosphere;
Reflux;
|
|
|
With
bis-triphenylphosphine-palladium(II) chloride; sodium carbonate;
In
tetrahydrofuran; water;
Reflux;
Inert atmosphere;
|
|
|
With
bis-triphenylphosphine-palladium(II) chloride; sodium carbonate;
In
tetrahydrofuran; water;
for 2h;
Inert atmosphere;
Reflux;
|
|
|
With
bis-triphenylphosphine-palladium(II) chloride; sodium carbonate;
In
tetrahydrofuran; water;
for 2h;
Reflux;
|
496.5 g |
|
With
bis-triphenylphosphine-palladium(II) chloride; sodium carbonate;
In
tetrahydrofuran;
at 80 ℃;
|
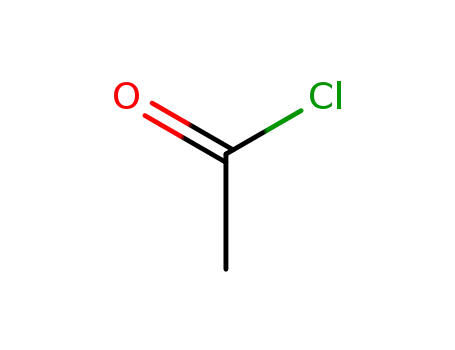
acetyl chloride

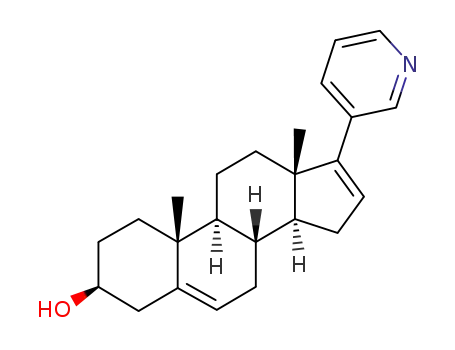
abiraterone


abiraterone acetate
| Conditions | Yield |
|---|---|
|
With
N-ethyl-N,N-diisopropylamine;
In
diethyl ether;
at 20 ℃;
for 4h;
|
85.1% |
|
With
2-(Dimethylamino)pyridine; triethylamine;
In
diethyl ether; ethanol; hexane; water;
|
84% |
|
With
triethylamine;
In
ethyl acetate;
at 5 - 20 ℃;
for 2h;
|
83% |
|
With
N-ethyl-N,N-diisopropylamine;
In
diethyl ether;
at 20 ℃;
for 3h;
|
83.18% |
|
With
triethylamine;
In
diethyl ether;
at 20 ℃;
for 3h;
Concentration;
|
81.8% |
|
With
pyridine;
In
dichloromethane;
|
70% |
|
With
2-(Dimethylamino)pyridine;
In
diethyl ether;
|
|
|
With
dmap; triethylamine;
In
dichloromethane;
at 20 - 30 ℃;
for 2h;
|
0.745 g |

3β-acetoxyandrosta-5,16-dien-17-yl trifluoromethanesulphonate

3-Diethylboranylpyridine
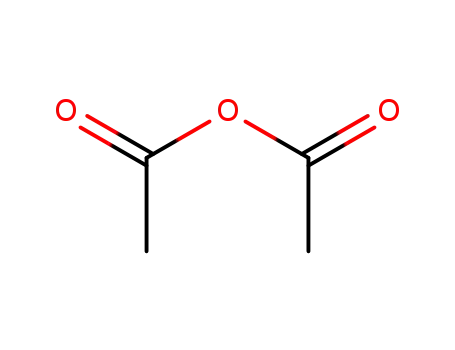
acetic anhydride

abiraterone
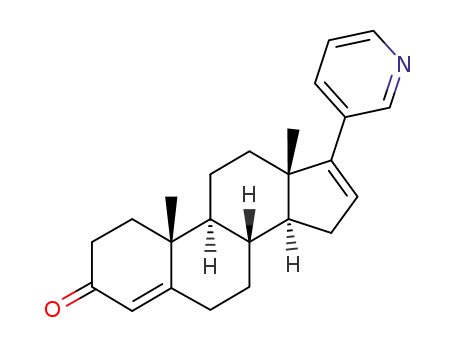
17-(3-pyridine)-4,16-dieneandrost-3-one

abiraterone