
Antioxidant BHT 264
CAS:128-37-0
Purity:99%
Contact Now
We will contact you as soon as possible
Your Location:Home >Products >Pharmaceutical API >98079-51-7

Product Details
|
Manufacturing Process |
A mixture of 1.00 g of 1-ethyl-6,7,8-trifluoro-1,4-dihydro-4-oxoquinoline-3- carboxylic acid, 1.10 g of 2-methylpiperazine and 10 ml of pyridine was heated for 15 minutes under reflux. The reaction mixture was evaporated and methanol was added to the residue. The precipitate was filtered and recrystallized from ethanol to give 0.36 g of the 1-ethyl-6,8-difluoro-1,4- dihydro-7-(3-methyl-1-piperazinyl)-4-oxoquinoline-3-carboxylic acid as colorless needles, melting point 239.0-240.5°C.By the usual manner the hydrochloride was prepared and recrystallized from water as colorless needles, melting point 290-300°C (decomp.). |
|
Therapeutic Function |
Antibacterial |
|
Pharmaceutical Applications |
A difluoropiperazinyl quinolone formulated as the hydrochloride salt for oral administration. The in-vitro activity is very similar to that of norfloxacin . It is active against Enterobacteriaceae and fastidious Gram-negative bacilli, including L. pneumophila. Activity against Campylobacter spp., Ps. aeruginosa, Acinetobacter and Chlamydia spp. is poor. It has reduced activity against staphylococci and poor activity against streptococci, L. monocytogenes, anaerobes and Mycobacterium spp.A 400 mg oral dose achieves a concentration of 3–5 mg/L after 1–1.5 h. In escalating oral doses of 100, 400 and 800 mg to volunteers, the AUC was essentially proportional to the dosage, the mean plasma concentrations following 100, 400 and 800 mg doses being approximately 1.1, 4.7 and 7.5 mg/L, respectively.Several metabolites have been described, accounting for <5% of the oral dose. Elimination occurs principally via the kidneys and 50–70% of a dose appears in the urine over 24 h. In patients with impaired renal function given 400 mg orally, the apparent elimination half-life ranged from 8 to 44 h, depending on the degree of renal failure. Non-renal clearance was also impaired, but there was no significant change in other pharmacokinetic parameters. The daily dosage (400 mg) should be reduced to 280 mg when the creatinine clearance falls below 30 mL/min. Hemodialysis has no effect on the plasma concentration. The effect of lomefloxacin on the plasma concentration of theophylline is clinically insignificant and no dosage adjustment is required.The main adverse event is phototoxicity; other adverse events (mainly diarrhea, abdominal pain, skin reactions, dizziness, headache and insomnia) occur in about 10% of patients.It is chiefly used in urinary tract infection, but is no longer widely available. |
|
Definition |
ChEBI: A fluoroquinolone antibiotic, used (generally as the hydrochloride salt) to treat bacterial infections including bronchitis and urinary tract infections. It is also used to prevent urinary tract infections prior to surgery. |
|
Brand name |
Uniquin |
InChI:InChI=1/C17H21F2N3O3/c1-3-21-8-11(17(24)25)16(23)10-6-12(18)15(13(19)14(10)21)22-5-4-20-9(2)7-22/h6,9,11,20H,3-5,7-8H2,1-2H3,(H,24,25)
The DL-lomenfloxacin hydrate is an inner...
The invention discloses a preparation me...
The invention relates to a method of pre...
The rate of the nucleophilic displacemen...
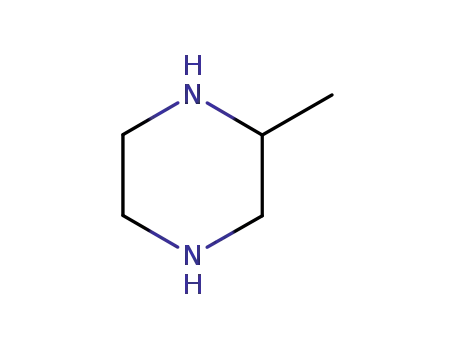
(RS)-2-methylpiperazine

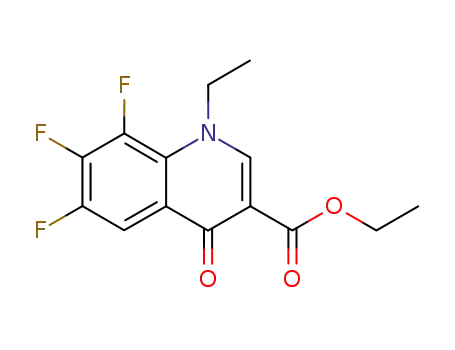
ethyl 1-ethyl-6,7,8-trifluoro-1,4-dihydro-4-oxoquinoline-3-carboxylate

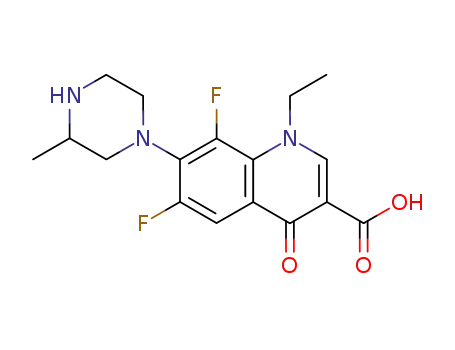
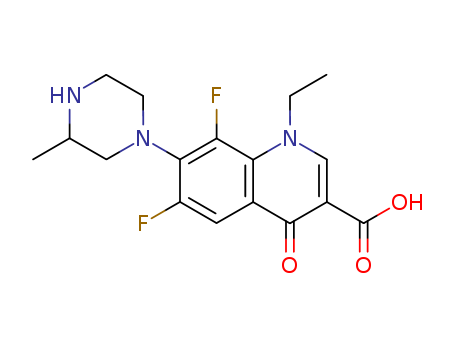
lomefloxacin
| Conditions | Yield |
|---|---|
|
(RS)-2-methylpiperazine; ethyl 1-ethyl-6,7,8-trifluoro-1,4-dihydro-4-oxoquinoline-3-carboxylate;
With
potassium carbonate;
In
water;
at 90 - 95 ℃;
for 10h;
With
hydrogenchloride; water;
at 20 ℃;
for 3h;
pH=7.5;
|
96% |
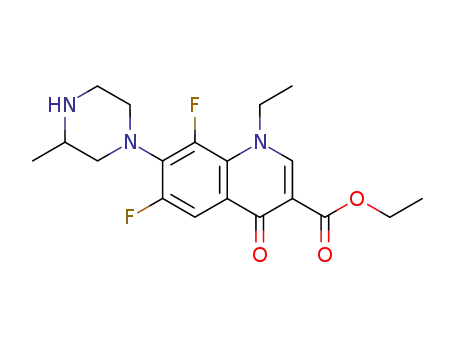
ethyl 1-ethyl-6,8-difluoro-1,4-dihydro-7-(3-methyl-1-piperazinyl)-4-oxoquinoline-3-carboxylate


lomefloxacin
| Conditions | Yield |
|---|---|
|
With
hydrogenchloride;
In
ethanol;
|
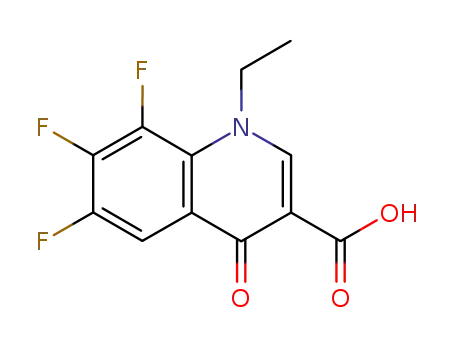
1-ethyl-6,7,8-trifluoro-1,4-dihydro-4-oxoquinoline-3-carboxylic acid

(RS)-2-methylpiperazine

ethyl 1-ethyl-6,8-difluoro-1,4-dihydro-7-(3-methyl-1-piperazinyl)-4-oxoquinoline-3-carboxylate

ethyl 1-ethyl-6,7,8-trifluoro-1,4-dihydro-4-oxoquinoline-3-carboxylate
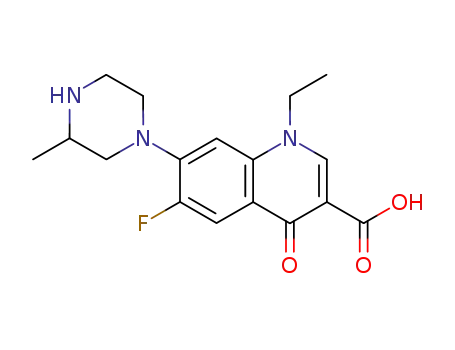
8-desfluoro-lomefloxacin
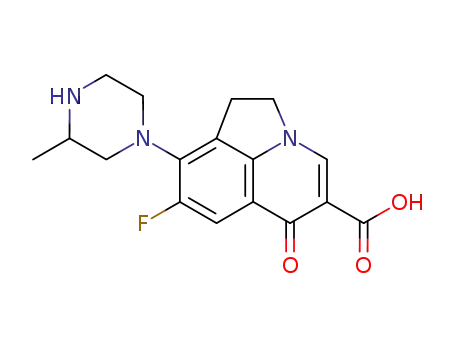
8-Fluoro-9-(3-methyl-piperazin-1-yl)-6-oxo-1,2-dihydro-6H-pyrrolo[3,2,1-ij]quinoline-5-carboxylic acid
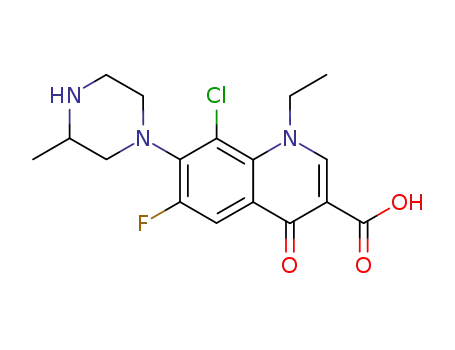
8-chloro-1-ethyl-6-fluoro-1,4-dihydro-7-(3-methyl-1-piperazinyl)-4-oxoquinoline-3-carboxylic acid