
Antioxidant BHT 264
CAS:128-37-0
Purity:99%
Contact Now
We will contact you as soon as possible
Your Location:Home >Products >Pharmaceutical API >107724-20-9
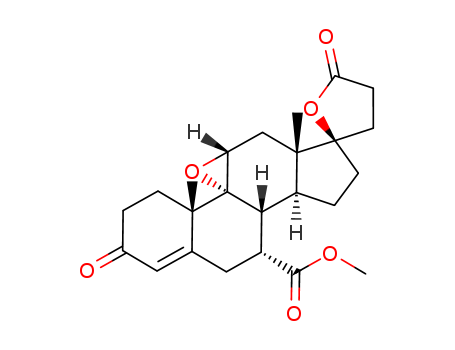

Product Details
|
Pharmacodynamics |
Eplerenone, an aldosterone receptor antagonist similar to spironolactone, has been shown to produce sustained increases in plasma renin and serum aldosterone, consistent with inhibition of the negative regulatory feedback of aldosterone on renin secretion. The resulting increased plasma renin activity and aldosterone circulating levels do not overcome the effects of eplerenone. Eplerenone selectively binds to recombinant human mineralocorticoid receptors relative to its binding to recombinant human glucocorticoid, progesterone and androgen receptors. |
|
Biological activity |
Eplerenone belongs to a class of drugs called aldosterone antagonists. A class of drugs is a group of medications that work in a similar way. These drugs are often used to treat similar conditions. Eplerenone works by interfering with the activity of a steroid in your body called aldosterone. Aldosterone acts to increase the amount of sodium and water you retain. This increased sodium and water can cause high blood pressure, which can in turn cause heart failure. |
|
Interactions |
Eplerenone is primarily metabolized by the cytochrome P450 enzyme CYP3A4. Thus the potential exists for adverse drug interactions with other drugs that induce or inhibit CYP3A4. Specifically, the concomitant use of the CYP3A4 potent inhibitors ketoconazole and itraconazole is contraindicated. Other CYP3A4 inhibitors including erythromycin, saquinavir, and verapamil should be used with caution. Other drugs that increase potassium concentrations may increase the risk of hyperkalemia associated with eplerenone therapy, including salt substitutes, potassium supplements and other potassium-sparing diuretics. |
|
Mechanism of action |
Aldosterone,with many physiological and pathological effects, can cause central blood pressure and endothelial injury (catecholamines enhance its role), reduce heart rate variability, induce ventricular arrhythmias, and promote retention of sodium, potassium and magnesium loss, promote myocardial fibrosis, necrosis and inflammation, damage the fibrinolytic system. Angiotensin converting enzyme inhibitors (also called angiotensin converting enzyme inhibitors, referred to as ACEI) and angiotensin Ⅱ receptor antagonist ARB aldosterone can inhibit the secretion of adrenaline, but after a period of treatment.The release of aldosterone was restored,which may even exceed the baseline plasma concentration levels. Despite adequate treatment of ACEI and ARB, aldosterone-induced damage can still happen, so it is necessary to use aldosterone receptor antagonists in the treatment of hypertension. Clinical studies have shown that patients who are not satisfied with the efficacy of ACEI or ARB therapy can add eplerenone along with the treatment. Non-selective aldosterone receptor antagonist spironolactone can reduce mortality in patients with congestive heart failure, However, the side effects of male hyperplasia and other diseases associated with sex hormones have limited its application in the treatment of hypertension. |
|
Adverse effects |
Common adverse drug reactions (ADRs) associated with the use of eplerenone include: hyperkalaemia, hypotension, dizziness, altered renal function, and increased creatinine concentration. |
|
Biological Activity |
Selective mineralocorticoid (aldosterone) receptor antagonist (IC 50 = 360 nM). Displays > 27-fold selectivity over androgen, progesterone and estrogen receptors (IC 50 > 10 μ M). Orally active antihypertensive in vivo . |
|
Biochem/physiol Actions |
Eplerenone is an aldosterone antagonist more specific for the mineralocorticoid receptor than spironolactone (S3378), having lower affinity for progesterone, androgen, and glucocorticoid receptors. |
|
Drug interactions |
Potentially hazardous interactions with other drugsACE inhibitors or AT-II antagonists: enhanced hypotensive effect; risk of severe hyperkalaemia.Anti-arrhythmics: concentration increased by amiodarone - reduce eplerenone dose.amiodarone - reduce eplerenone dose. Antibacterials: concentration increased by clarithromycin and telithromycin - avoid; concentration increased by erythromycin - reduce eplerenone dose; concentration reduced by rifampicin - avoid; avoid with lymecycline; increased risk of hyperkalaemia with trimethoprim.Antidepressants: concentration reduced by St John’s wort - avoid; increased risk of postural hypotension with tricyclics; enhanced hypotensive effect with MAOIs.Antiepileptics: concentration reduced by carbamazepine, fosphenytoin, phenytoin, phenobarbital and primidone - avoid.Antifungals: concentration increased by itraconazole and ketoconazole - avoid; concentration increased by fluconazole - reduce eplerenone dose.Antihypertensives: enhanced hypotensive effect, increased risk of first dose hypotensive effect with post-synaptic alpha-blockers.Antivirals: concentration increased by ritonavir - avoid; concentration increased by saquinavir - reduce eplerenone doseCiclosporin: increased risk of hyperkalaemia and nephrotoxicityCytotoxics: increased risk of nephrotoxicity and ototoxicity with platinum compounds.NSAIDs: increased risk of hyperkalaemia (especially with indometacin); increased risk of nephrotoxicity; antagonism of diuretic effect.Potassium salts: increased risk of hyperkalaemia.Lithium: reduced lithium excretion - avoidTacrolimus: increased risk of hyperkalaemia and nephrotoxicity.CYP3A4 inhibitors: Do not exceed a dose of 25 mg daily for eplerenone.CYP3A4 inducers: reduced eplerenone concentration - avoid. |
|
Metabolism |
Eplerenone metabolism is primarily mediated via CYP3A4. No active metabolites of eplerenone have been identified in human plasma. Less than 5% of an eplerenone dose is recovered as unchanged drug in the urine and faeces. Following a single oral dose of radiolabelled drug, approximately 32% of the dose was excreted in the faeces and approximately 67% was excreted in the urine |
|
Overview |
Eplerenone is in a class of medications called mineralocorticoid receptor antagonists. It can be used individually or in combination with other medications to treat hypertension by blocking the action of aldosterone, a natural substance in the body that raises blood pressure. |
|
Chemical properties |
Eplerenone is an odorless, white to off-white crystalline powder. It is very slightly soluble in water, with its solubility essentially pH-independent. The octanol/water partition coefficient of eplerenone is approximately 7.1 at pH 7.0. |
|
Brand name |
Inspra (Searle). |
|
General Description |
Eplerenone, 9,11α-epoxy-17α-hydroxy-3-oxopregn-4-ene-7α,21-dicarboxylic acid, γ-lactone,methyl ester (Inspra), is a newer aldosterone antagonist that isused for the treatment of hypertension. |
InChI:InChI=1/C24H30O6/c1-21-7-4-14(25)10-13(21)11-15(20(27)28-3)19-16-5-8-23(9-6-18(26)30-23)22(16,2)12-17-24(19,21)29-17/h10,15-17,19H,4-9,11-12H2,1-3H3/t15-,16+,17-,19+,21+,22+,23-,24-/m1/s1
A novel and efficient method of stereose...
Two unknown impurities were observed dur...
The invention discloses a method for syn...
The invention discloses a preparation me...
The invention relates to a canrenone der...
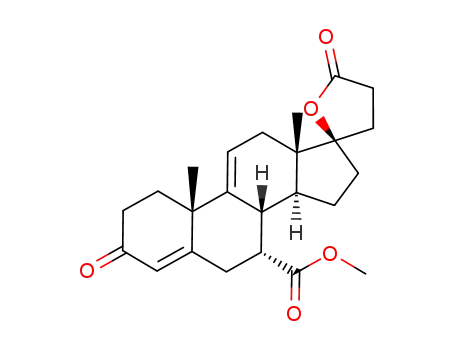
17α-pregna-4,9(11)-diene-7α,21-dicarboxylic acid-17β-hydroxy-3-oxo-γ-lactone-7-methyl ester

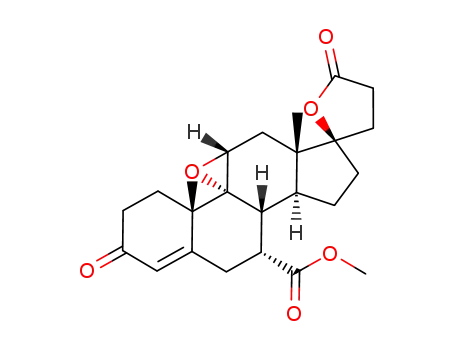
eplerenone
| Conditions | Yield |
|---|---|
|
With
dipotassium hydrogenphosphate; trichloroacetamide; dihydrogen peroxide;
In
dichloromethane;
at 0 - 15 ℃;
for 24h;
pH=9;
|
98% |
|
With
trichloroacetamide; dihydrogen peroxide; potassium acetate;
In
dichloromethane;
at 10 - 15 ℃;
for 0.75h;
Temperature;
|
91.3% |
|
With
potassium phosphate; trichloroacetamide; dihydrogen peroxide;
In
dichloromethane;
at 20 ℃;
|
85.3% |
|
With
dipotassium hydrogenphosphate; trichloroacetamide; dihydrogen peroxide;
In
dichloromethane; water;
at 20 - 31 ℃;
for 7.5 - 12.6h;
Product distribution / selectivity;
|
82% |
|
With
dihydrogen peroxide; trichloroacetic acid anhydride;
In
dichloromethane;
for 1.5h;
below 4 deg C;
|
71.6% |
|
With
dipotassium hydrogenphosphate; trichloroacetamide; dihydrogen peroxide;
In
dichloromethane; water;
at 25 ℃;
for 18.5h;
|
65.8% |
|
With
dipotassium hydrogenphosphate; trichloroacetamide; dihydrogen peroxide;
In
dichloromethane; water;
at 20 ℃;
|
|
|
With
dipotassium hydrogenphosphate; trichloroacetamide; dihydrogen peroxide;
In
dichloromethane; water;
at 15 - 28 ℃;
for 4 - 18h;
|
3 - 5.9 g |
|
With
dipotassium hydrogenphosphate; trichloroacetamide; dihydrogen peroxide;
In
dichloromethane; water;
at 27 ℃;
for 5h;
|
|
|
With
dipotassium hydrogenphosphate; trichloroacetamide; dihydrogen peroxide;
In
toluene;
at 28 ℃;
for 4h;
|
2.5 g |
|
With
dipotassium hydrogenphosphate; 2-chloro-2,2-difluoroacetamide; dihydrogen peroxide;
In
dichloromethane; water;
at 25 ℃;
for 23h;
|
|
|
With
dipotassium hydrogenphosphate; heptafluorobutyramide; dihydrogen peroxide;
|
|
|
With
dipotassium hydrogenphosphate; trichloroacetamide; dihydrogen peroxide;
In
dichloromethane;
at 10 - 20 ℃;
|
|
|
With
disodium hydrogenphosphate; trichloroacetamide; dihydrogen peroxide;
In
dichloromethane;
at 15 - 20 ℃;
for 18h;
|
5.2 g |
|
With
dipotassium hydrogenphosphate; 2-chloro-2,2-difluoroacetamide; dihydrogen peroxide;
In
toluene;
at 55 ℃;
Reagent/catalyst;
Temperature;
Solvent;
|
5.5 g |
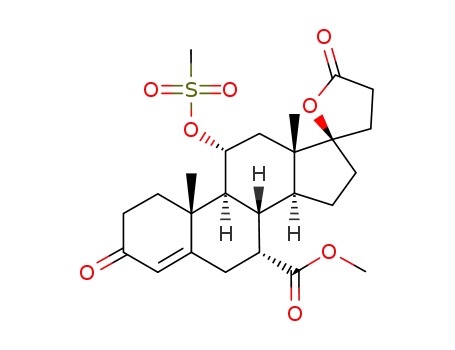
methyl hydrogen 17α-hydroxy-11α-(methylsulfonyl)oxy-3-oxopregn-4-ene-7α,21-dicarboxylate, γ-lactone


eplerenone
| Conditions | Yield |
|---|---|
|
With
dipotassium hydrogenphosphate; dihydrogen peroxide;
|
82% |
|
Multi-step reaction with 2 steps
1: potassium formate; formic acid / acetic anhydride / 100 °C
2: trichloroacetamide; dihydrogen peroxide; dipotassium hydrogenphosphate / dichloromethane / 10 - 20 °C
With
dipotassium hydrogenphosphate; formic acid; trichloroacetamide; dihydrogen peroxide; potassium formate;
In
dichloromethane; acetic anhydride;
|

17α-pregna-4,9(11)-diene-7α,21-dicarboxylic acid-17β-hydroxy-3-oxo-γ-lactone-7-methyl ester
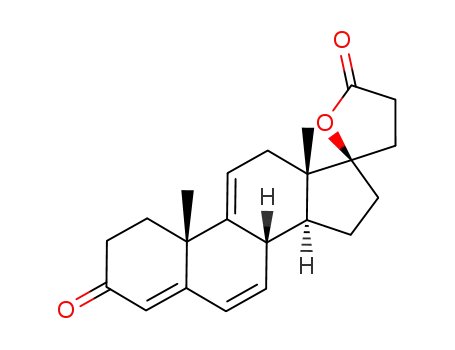
Δ9(11)-canrenone
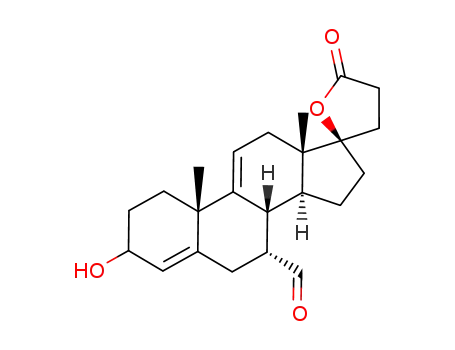
C23H30O4
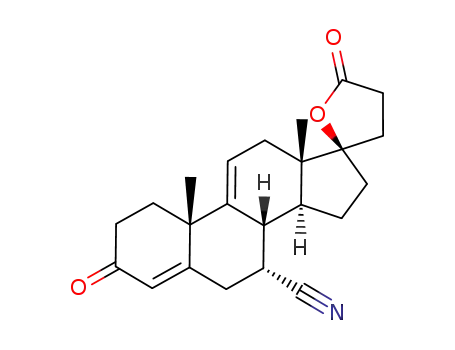
17β-hydroxy-7α-cyano-pregna-4,9(11)-dien-3-one-21-carboxylic acid γ-lactone