
Antioxidant BHT 264
CAS:128-37-0
Purity:99%
Contact Now
We will contact you as soon as possible
Your Location:Home >Products >Intermediates >86-87-3
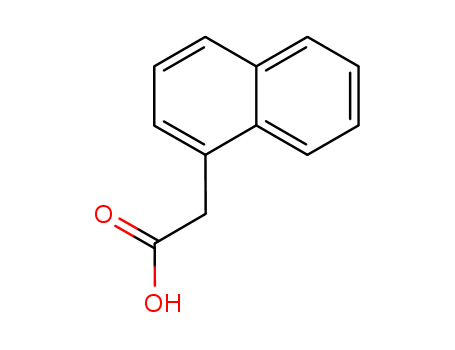

Product Details
|
Biochem/physiol Actions |
1-Naphthaleneacetic acid is one of the synthetic auxins, used in plant propagation. It can induce the formation of lateral and adventitious roots. |
|
Hazard |
Skin irritant. |
|
Trade name |
AGRONAA?; ALCO? NAA; ALPHASPRA ?; AMCOTONE? APPL-SET?; CELMONE?; DESTRUXOL?; DIP’N GROW?; FRUITONE?; GOLDENGRO?; HORMEX?; KLINGTITE?; LIQUISTIK ?; NAA 800?; NAFUSAKU?; NIAGARASTIK ?; NU-TONE?; PARMONE?; PHYMONE?; PIMACOL-SOL?; PLANOFIX?; PLUCKER?; PRIMACOL?; RHIZOPON B ROOTING POWDER; ROOTONE? (component, with Indole-3-butyric acid and 1-Naphthaleneacetamide); STAFAST?; STIK?; STOPDROP ?; TEKKAM?; TIPOFF?; TRANSPLANTONE? (component, with 1-Naphthaleneacetamide); TREHOLD ?; VARDHAK? |
|
Safety Profile |
Poison by intraperitoneal route. Moderately toxic by ingestion. Mutation data reported. A skin, mucous membrane, and severe eye irritant. Can cause depression. A pesticide, When heated to decomposition it emits acrid smoke and irritating fumes. |
|
Potential Exposure |
1-Naphthaleneacetic acid is a carboxylic acid plant growth regulator used for thinning fruit sets in apples, pears, olives, and some citrus. Induces root formation on cuttings and transplants. Inhibits fruit drops. Not currently registered in EU countries (may be pending). |
|
Shipping |
UN1759 Corrosive solids, n.o.s., Hazard Class: 8; Labels: 8-Corrosive material, Technical Name Required. |
|
Purification Methods |
Crystallise the acid from EtOH or water. [Beilstein 9 H 666, 9 IV 2424.] |
|
Incompatibilities |
Incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, strong bases, strong acids, oxoacids, epoxides, chlorates nitrates, ammonia, aliphatic amines, alkanolamines, isocyanates, alkylene oxides, epichlorohydrin. Compounds of the carboxyl group react with all bases, both inorganic and organic (i.e., amines) releasing substantial heat, water and a salt that may be harmful. Incompatible with arsenic compounds (releases hydrogen cyanide gas), diazo compounds, dithiocarbamates, isocyanates, mercaptans, nitrides, and sulfides (releasing heat, toxic, and possibly flammable gases), thiosulfates and dithionites (releasing hydrogen sulfate and oxides of sulfur). |
|
Waste Disposal |
Incineration. In accordance with 40CFR165, follow recommendations for the disposal of pesticides and pesticide containers. |
|
uses |
1-Naphthaleneacetic acid is widely employed in agriculture as a synthetic plant hormone in the auxin family or a plant growth regulator, which can find applications in tissue culture.1-Naphthaleneacetic acid (NAA) is a synthetic phytohormone auxin that is added to cell culture media such as Murashige & Skoog media and Chu′s N6 media.1-Naphthaleneacetic acid has been used:to modify MS media for the initiation of callusas a supplement in solid K3 medium for the selection of transformantsin the preparation of culture medium like V-KM medium and Cg medium |
|
Definition |
1-Naphthaleneacetic acid is a naphthylacetic acid substituted by a carboxymethyl group at position 1. |
|
General Description |
1-Naphthaleneacetic acid is widely employed in agriculture as a synthetic plant hormone in the auxin family or a plant growth regulator, which can find applications in tissue culture. |
|
Agricultural Uses |
Plant growth regulator: An agent for thinning fruit sets in apples, pears, olives and some citrus. Induces root formation on cuttings and transplants. Inhibits fruit drops. Not currently registered in EU countries (pending). Registered for use in the U.S. and Canada. |
InChI:InChI=1/C12H10O2/c13-12(14)8-10-6-3-5-9-4-1-2-7-11(9)10/h1-7H,8H2,(H,13,14)/p-1
-
The degradation and mineralization of th...
-
-
Free radical-induced oxidation reactions...
The invention discloses C labeled α - na...
Electrocarboxylation of organic halides ...
Hydrolysis of amides to carboxylic acids...
Tandem Meinwald rearrangement and nucleo...
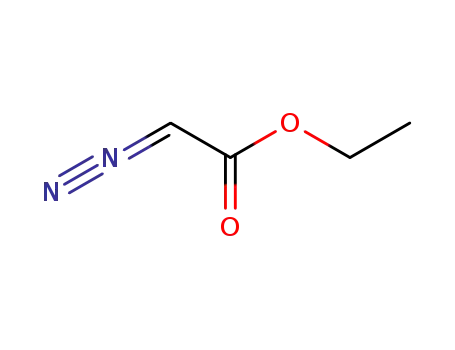
diazoacetic acid ethyl ester

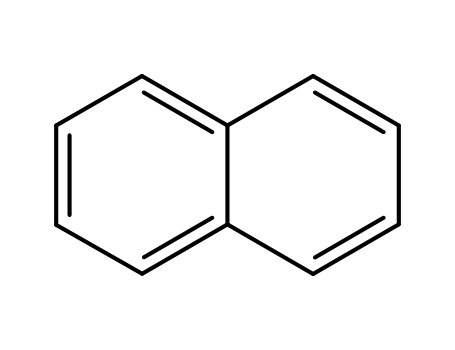
naphthalene

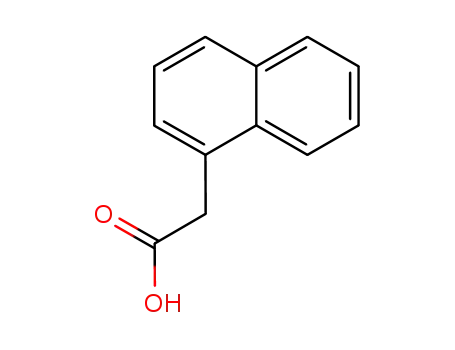
naphth-1-yl acetic acid

![1a,7b-dihydro-1H-cyclopropa[a]naphthalene-1-exo-carboxylic acid](/upload/2025/1/23eb50a1-3744-48b8-88cd-810624f80867.png)
1a,7b-dihydro-1H-cyclopropa[a]naphthalene-1-exo-carboxylic acid

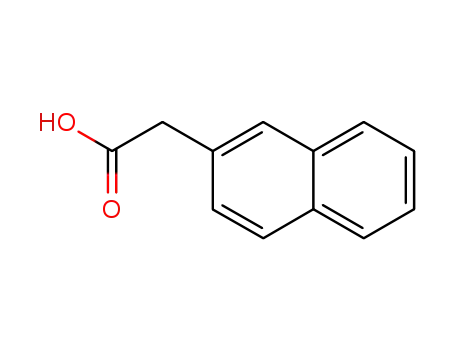
2-naphthylacetic acid
| Conditions | Yield |
|---|---|
|
tetrakis(trifluoroacetato)rhodium(II);
In
dichloromethane;
for 2.25h;
|
![3-methoxy-2-[1]naphthyl-acrylic acid ethyl ester](/upload/2025/1/ca4dc548-04c4-45c2-9a75-8e767d2258c3.png)
3-methoxy-2-[1]naphthyl-acrylic acid ethyl ester

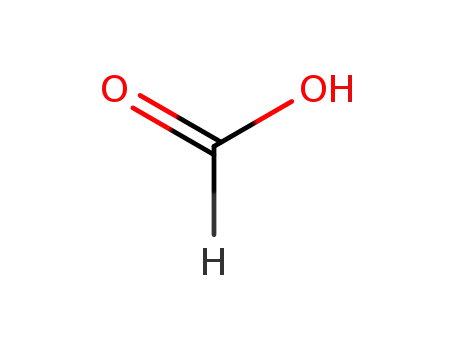
formic acid


naphth-1-yl acetic acid
| Conditions | Yield |
|---|---|
|
|

naphthalen-1-ylacetonitrile
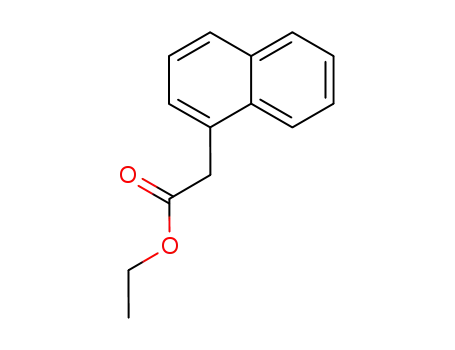
ethyl 2-(1-naphthyl)acetate
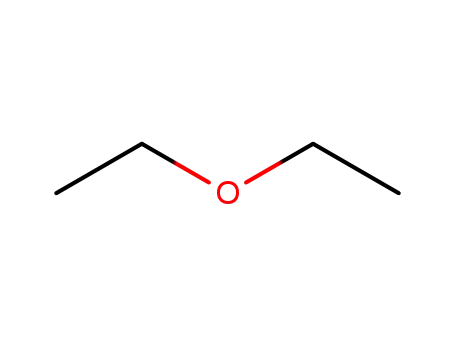
diethyl ether
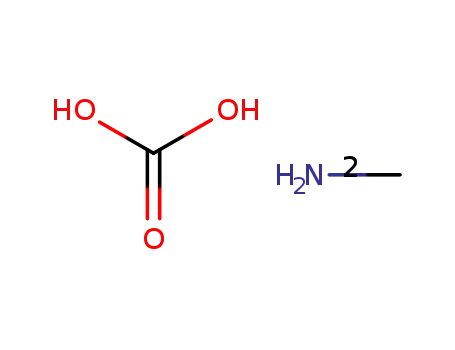
methylammonium carbonate
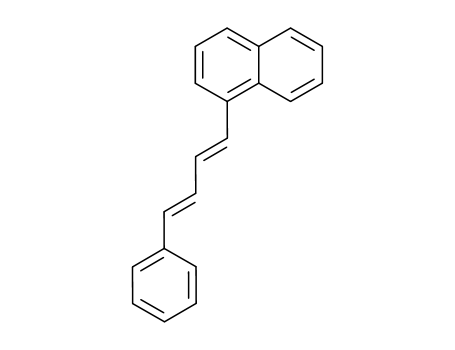
1-(1-naphthyl)-4-phenyl-1,3-butadiene
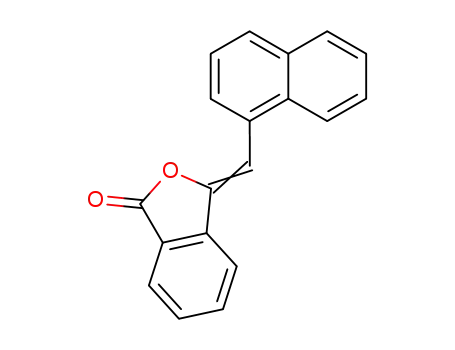
3-naphthalen-1-ylmethylene-3H-isobenzofuran-1-one
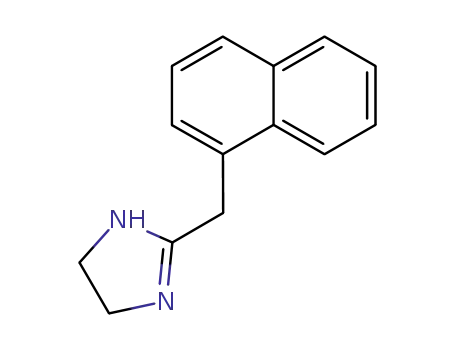
naphazoline
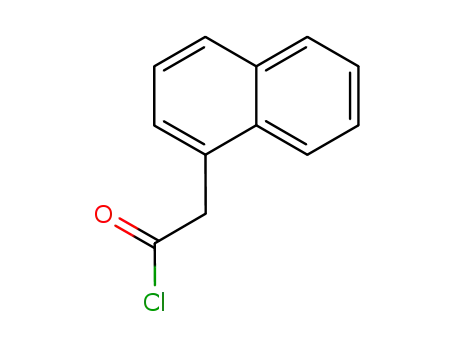
naphthalen-1-yl-acetyl chloride