
Antioxidant BHT 264
CAS:128-37-0
Purity:99%
Contact Now
We will contact you as soon as possible
Your Location:Home >Products >Pharmaceutical API >10101-63-0


Product Details
|
Physical properties |
Yellow hexagonal crystals; density 6.16 g/cm3; melts at 402°C; vaporizes at 954°C; decomposes at 180°C when exposed to green light; slightly soluble in water (0.44 g/L at 0°C and 0.63 g/L at 20°C); Ksp 8.49x10-9 at 25°C; partially soluble in boiling water (4.1 g/L at 100°C); insoluble in ethanol; soluble in alkalis and alkali metal iodide solutions. |
|
Preparation |
Lead diiodide is prepared by mixing aqueous solutions of lead nitrate or lead acetate with an aqueous solution of potassium or sodium iodide or hydriodic acid, followed by crystallization. The product is purified by recrystallization. Pb2+(aq) + 2Iˉ (aq) → PbI2(s). |
|
General Description |
A yellow crystalline solid. Insoluble in water and denser than water. Primary hazard is threat to the environment. Immediate steps should be taken to limit spread to the environment. Used in printing and photography, to seed clouds and other uses. |
|
Air & Water Reactions |
Slightly water soluble. |
|
Reactivity Profile |
Lead(II) iodide has weak oxidizing or reducing powers. Redox reactions can however still occur. The majority of compounds in this class are slightly soluble or insoluble in water. If soluble in water, then the solutions are usually neither strongly acidic nor strongly basic. These compounds are not water-reactive. Light sensitive |
|
Hazard |
Lead diiodide is toxic if ingested. The symptoms are those of lead poisoning. |
|
Health Hazard |
Early symptoms of lead intoxication via inhalation or ingestion are most commonly gastrointestinal disorders, colic, constipation, etc.; weakness, which may go on to paralysis, chiefly of the extensor muscles of the wrists and less often the ankles, is noticeable in the most serious cases. Ingestion of a large amount causes local irritation of the alimentary tract. Pain, leg cramps, muscle weakness, paresthesias, depression, coma, and death may follow in 1 or 2 days. Contact with eyes causes irritation. |
|
Potential Exposure |
Lead iodide is used in bronzing, gold pencils; mosaic gold; printing, and photography |
|
Shipping |
UN3288 Toxic solids, inorganic, n.o.s., Hazard Class: 6.1; Labels: 6.1-Poisonous materials, Technical Name Required. UN3077 Environmentally hazardous substances, solid, n.o.s., Hazard class: 9; Labels: 9-Miscellaneous hazardous material, Technical Name Required |
|
Purification Methods |
It crystallises from a large volume of water. The solubility in H2O is 1.1% at ~10o, and 3.3% at ~ 100o. |
|
Incompatibilities |
Lead iodide has weak oxidizing or reducing powers. Redox reactions can however still occur. The majority of compounds in this class are slightly soluble or insoluble in water. If soluble in water, then the solutions are usually neither strongly acidic nor strongly basic. These compounds are not water-reactive. Light sensitive Contact with oxidizers or active metals may cause violent reaction |
InChI:InChI=1/2HI.Pb.4H/h2*1H;;;;;/q;;+2;;;;/p-2/r2HI.H4Pb/h2*1H;1H4/q;;+2/p-2
We report the cooling-induced crystalliz...
Depositing a pinhole-free perovskite fil...
Lead iodide (PbI2) clusters were synthes...
The synthesis of guanidinium lead iodide...
We have measured resonant Raman specta a...
We report the results obtained in four d...
Lead iodide (PbI2) films/crystals with v...
Reverse micelle solutions can be used fo...
A novel black organoammonium iodoplumbat...
129I Moessbauer spectroscopy was applied...
The structure of the hybrid perovskite H...
Nanoparticles of a new PbII metal-organi...
High-purity lead iodide (PbI2) is prepar...
-
To date, the formation mechanism of orga...
Hybrid organic-inorganic perovskites hav...
The series of trimethylplatinum(IV) comp...
PbI2 microcrystallites are embedded into...
Over the past decade, remarkable progres...
The development of two-dimensional nanom...
Novel lead and bismuth dipyrido complexe...
Organo-lead halide perovskite solar cell...
We report the pressure-induced crystallo...
The reactions products of tertiary arsin...
Lead iodide (PbI2) nanostructures have b...
Microwave-assisted synthesis of pure and...
Lead halides (PbCl2, PbBr2 and PbI2) of ...
Thin films of the methylammonium lead ha...
A novel lead(II) cluster [Pb7(μ3-O)(μ4-O...
The compound Pb2PO4I was synthesized via...
PbI2 forms iodo-bridged neutral polymer ...
Dielectric properties determine by elect...
Nanoparticles of two new 0D, lead(II) co...
The growth of PbI2 precipitates on singl...
Hole conductivity values in compact and ...
The synthesis of hydroxylammonium lead i...
A novel 2D layered iodoplumbate, [Ni(opd...
Composite films of polyvinyl alcohol/lea...
Perovskite solar cells (PSCs), with thei...
lead(II) oxide

aluminium(III) iodide

aluminum oxide

lead(II) iodide
| Conditions | Yield |
|---|---|
|
24h at 230°C;;
|
|
|
24h at 230°C;;
|
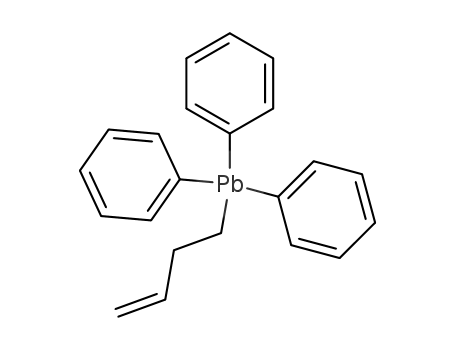
3-butenyltriphenylplumbane

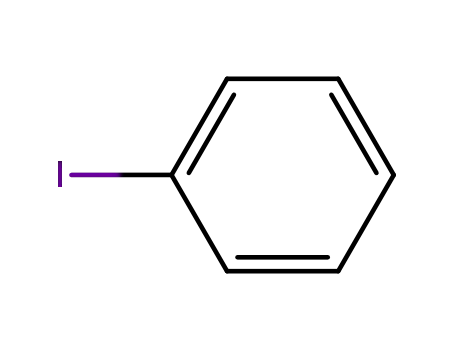
iodobenzene

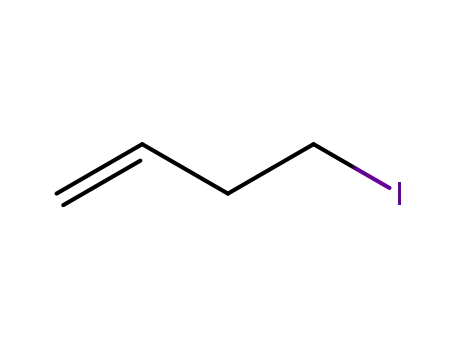
4-iodobut-1-ene

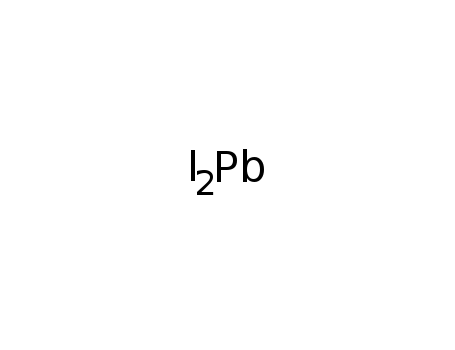
lead(II) iodide
| Conditions | Yield |
|---|---|
|
With
I2;
In
chloroform-d1;
(N2), stirred, treated with 3 equiv I2 at 25°C; filtered; NMR, IR, mass. spectra;
|
93% 96% 85% |
iodine
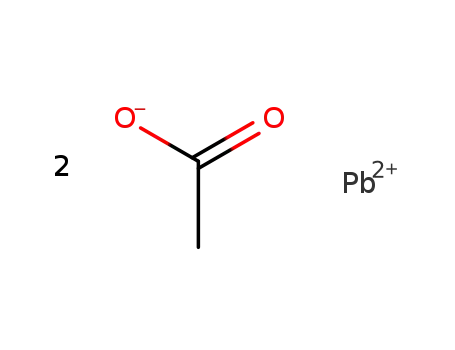
lead acetate
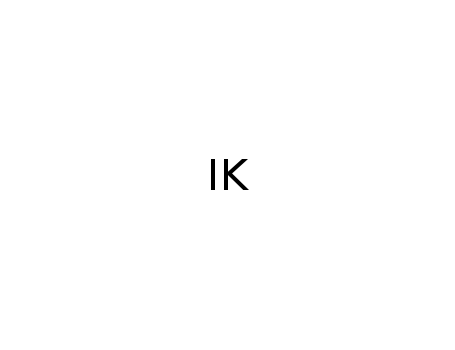
potassium iodide

lead
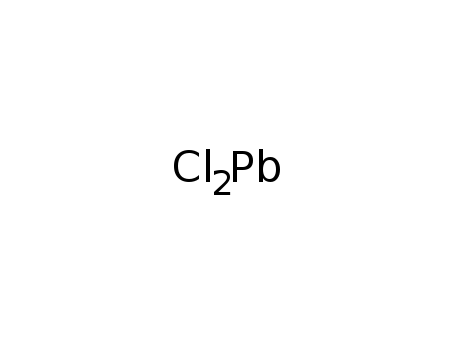
lead(II) chloride
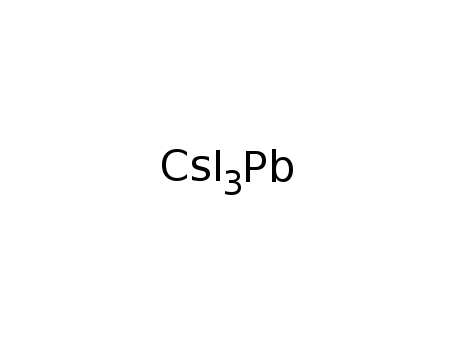
CsI3Pb, black
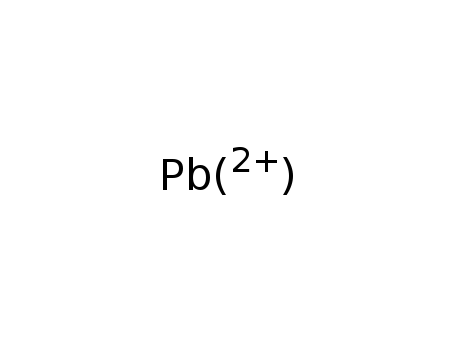
lead(2+) cation
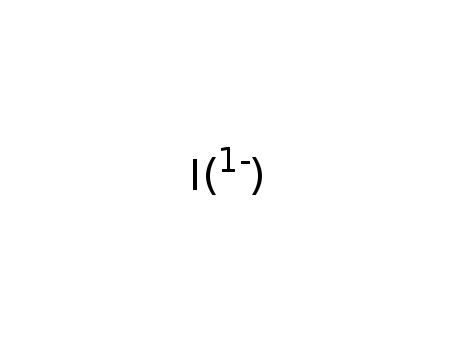
iodide