
Antioxidant BHT 264
CAS:128-37-0
Purity:99%
Contact Now
We will contact you as soon as possible
Your Location:Home >Products >Pharmaceutical API >42835-25-6


Product Details
|
Preparation |
Synthesis: Condensation of 5-fluoro-2-methyltetrahydroquinoline with diethyl ethoxy-methylenemalonate followed by thermal cycli- zation gives ethyl 6,7-dihydro-9-fluoro-5-meth-yl-1-oxo-1H,5H-benzo[i,j]quinolizine-2-car-boxylate,which is saponified with sodium hydroxide to give flumequine. |
|
Manufacturing Process |
6-Fluoro-2-methyltetrahydroquinoline (32.2 g, 0.2 mol) is mixed with diethyl ethoxymethylenemalonate, and the mixture is heated at 125°C to 130°C for 3 hours. Polyphosphoric acid (200 g) is added, and the solution is gradually heated to 115°C to 120°C in an oil bath with occasional stirring. The temperature is maintained for 1 hour, then the mixture is poured into 600 ml of water and neutralized with 40% sodium hydroxide solution. The product ester which precipitates is separated by filtration, washed with water and suspended in 2 liters of 10% sodium hydroxide solution. The mixture is heated on the steam bath for 1 hour, treated with decolorizing charcoal, filtered, then neutralized with concentrated hydrochloric acid. The solid product is isolated by filtration of the hot solution, washed with water and recrystallized from dimethylformamide. |
|
Therapeutic Function |
Antibacterial |
|
Pharmaceutical Applications |
A tricyclic fluorinated 4-quinolone, with activity similar to that of nalidixic acid in vitro, although it is somewhat more active against some Enterobacteriaceae. Following escalating oral doses of 400, 800 or 1200 mg, mean peak plasma levels reached at 2 h are 13.5, 23.8 and 31.9 mg/L, respectively. The apparent elimination half-life is about 7 h. The main metabolite, hydroxyflumequine, is much more rapidly eliminated. About 60% of a dose appears in the urine, mostly in the form of conjugates. Urinary concentrations following an 800 mg dose are 10–35 mg/L, with a peak of 105 mg/L. It has no effect on the pharmacokinetics of theophylline.Flumequine is generally well tolerated, side effects being mainly mild gastrointestinal tract disturbances, rashes, dizziness and confusion.It is principally used in uncomplicated urinary tract infections. |
|
Definition |
ChEBI: Flumequine is a member of the class of pyridoquinolines that is 1-oxo-6,7-dihydro-1H,5H-pyrido[3,2,1-ij]quinoline carrying additional carboxy, methyl and fluoro substituents at positions 2, 5 and 9 respectively. It is a pyridoquinoline, a 3-oxo monocarboxylic acid, an organofluorine compound and a quinolone antibiotic. |
InChI:InChI=1/C14H12FNO3/c1-7-2-3-8-4-9(15)5-10-12(8)16(7)6-11(13(10)17)14(18)19/h4-7H,2-3H2,1H3,(H,18,19)
Cobalt oxide/cobalt-based nanoparticles ...
The antibacterial agent 9-fluoro-6,7-dih...
Process for preparing 6,7-dihydro-9-fluo...
An improved process for preparing the an...
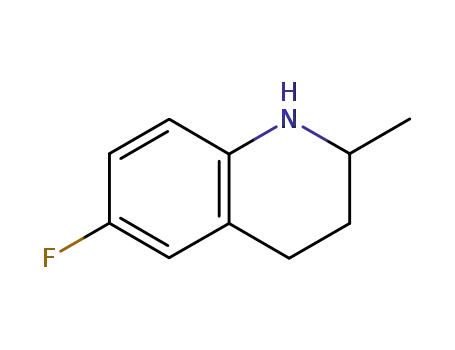
6-fluoro-1,2,3,4-tetrahydro-2-methylquinoline

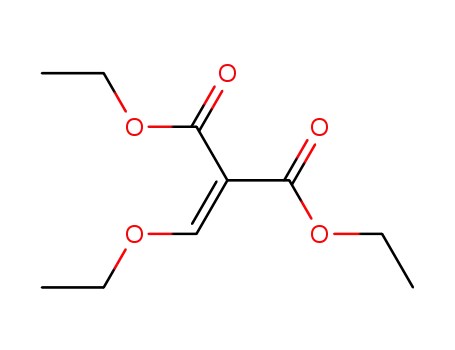
diethyl 2-ethoxymethylenemalonate

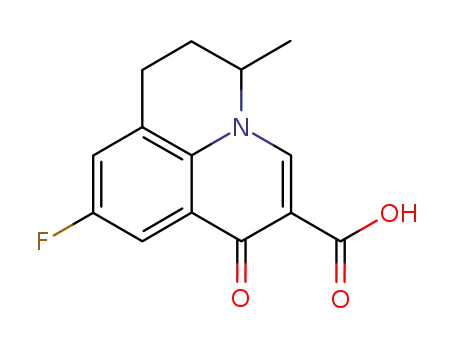
flumequine
| Conditions | Yield |
|---|---|
|
With
PPA;
In
water; toluene;
|
|
|
In
sodium hydroxide; toluene;
|
|
|
With
PPA;
In
water; toluene;
|
|
|
In
sodium hydroxide; toluene;
|
![5-[1-(6-fluoro-2-methyl-1,2,3,4-tetrahydroquinolyl)]-methylene-2,2-dimethyl-1,3-dioxan-4,6-dione](/upload/2025/1/7f5e24c7-5a10-484e-b0cc-8b574b5a2cc0.png)
5-[1-(6-fluoro-2-methyl-1,2,3,4-tetrahydroquinolyl)]-methylene-2,2-dimethyl-1,3-dioxan-4,6-dione


flumequine
| Conditions | Yield |
|---|---|
|
With
sodium hydroxide; PPA;
In
5,5-dimethyl-1,3-cyclohexadiene;
|
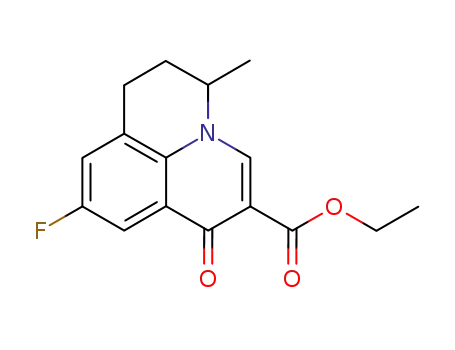
9-fluoro-5-methyl-1-oxo-6,7-dihydro-1H,5H-pyrido[3,2,1-ij]quinoline-2-carboxylic acid ethyl ester

6-fluoro-1,2,3,4-tetrahydro-2-methylquinoline
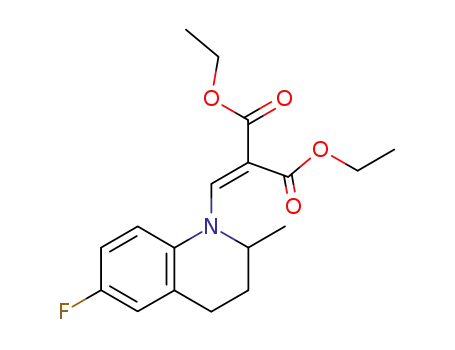
Diethyl (2-methyl-6-fluorotetrahydro-quinolin-1-yl)methylenemalonate

diethyl 2-ethoxymethylenemalonate
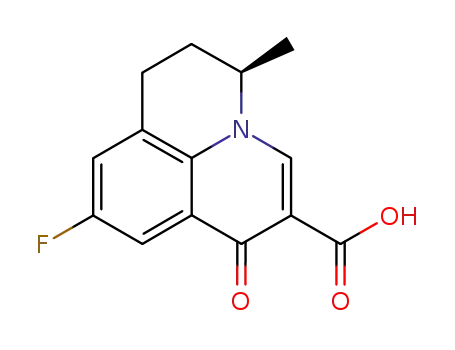
R-(+)-flumequine
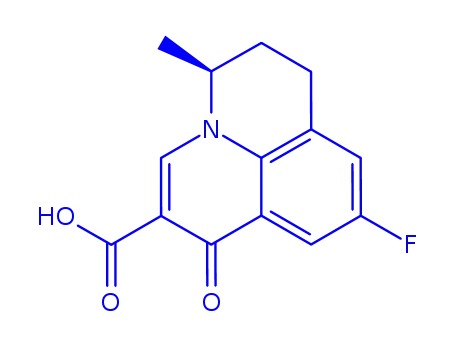
S-(-)-flumequine

9-fluoro-5-methyl-1-oxo-6,7-dihydro-1H,5H-pyrido[3,2,1-ij]quinoline-2-carboxylic acid ethyl ester